Abstract
Introduction:
Breast cancer is a leading cause of morbidity and mortality among women. Advances in molecular biology have improved detection and treatment, but conventional histopathological factors remain crucial for prognosis. Tumour budding, defined as clusters of less than 5 tumour cells detached from the main tumour, has been linked to poor prognosis in several cancers. This study explores the association between intra-tumoral budding (ITB) and peripheral tumour budding (PTB) with known prognostic factors in Invasive Breast Carcinoma of no special type (IBC NST).
Materials and methods:
This retrospective study analysed 70 cases of IBC NST diagnosed at Kasturba Medical College, Manipal, between January 2020 and December 2021. Tumour budding was classified as high-grade or low-grade based on density, which denotes the number of buds per x20 field. Clinicopathological data, including hormone receptor status, Ki-67 index, lymphovascular invasion (LVI), perineural invasion (PNI), and axillary lymph node involvement, were obtained. Statistical analyses were performed to identify a significant association between tumour budding and these factors. Univariate and multivariate logistic regression analyses were also done to demonstrate the significance of association.
Results:
High-grade PTB showed significant associations with LVI (p = 0.046), PNI (p = 0.017), and axillary lymph node involvement (p = 0.021). In contrast, high-grade ITB was only significantly correlated with axillary lymph node involvement (p = 0.044). LVI (p-value = 0.240) and axillary lymph node involvement (p-value = 0.142) did not show any association with PTB on multivariate analysis and PNI (p-value = 0.074) near significant association with PTB). A significant inverse association was observed between PTB and Ki-67 (p = 0.012), which remained significant in univariate and multivariate analysis (p-value = 0.017). No significant associations were found between tumour budding and hormone receptor status or menopausal status.
Conclusion:
Peripheral tumour budding (PTB) is significantly associated with several poor prognostic factors in IBC NST, while intra-tumoral budding (ITB) correlates primarily with axillary lymph node involvement. Tumor budding, particularly PTB, could serve as an important prognostic marker in breast cancer. Further research is needed to standardize tumour budding assessment in clinical practice.
Introduction
Breast cancer is one of the most highly prevalent malignancies in women and causes considerable morbidity and mortality [1]. There were an estimated 20 million new breast cancer cases worldwide and 9.7 million cancer deaths in 2022, with the majority of them being in Asia [2]. The advances in genetics and molecular biology have improved the detection and treatment of breast carcinomas, which in turn have led to better outcomes. However, conventional histopathological prognostic parameters still play a crucial role in prognostication of breast cancers. Hence, identifying more meaningful and reliable histopathological factors to complement the current evaluation protocols is essential, and this is where tumour budding becomes relevant [3, 4].
Tumour budding is a pathological phenomenon associated with many cancers. Its definition varies from study to study but generally is defined as a cluster of 5 or fewer tumour cells which have detached from the bulk of the tumour and which don’t show features of differentiation [5]. They can be observed at the invasive margins of the tumour and are called peritumoral or peripheral tumour buds (PTB), while those that are seen in the tumour mass are called intra-tumoral buds (ITB) [6, 7].
At the molecular level, they are hypothesised to be the histological manifestations of epithelial-mesenchymal transition (EMT). EMT is thought to be a multi-step, dynamic phenomenon that occurs in epithelial cells, where they lose their ability to adhere to their neighbouring cell and develop migratory and invasive characteristics like mesenchymal cells [8]. Both EMT and its opposite process, mesenchymal-epithelial transition (MET), are physiological processes that are important for tissue repair, wound healing and embryonic development. The abnormal activation of EMT is now recognized as a key characteristic of cancer metastasis [8–10]. Hence, tumour budding was also thought to be a general invasive indicator and a poor prognostic factor.
The association between tumour budding with cancers were first described in 1949 by Imai with respect to gastric cancers [11]. Now, tumour budding is recognised as an aggressive and prognostic indicator in colorectal, oesophageal and gastric cancers [3, 12]. However, there is limited information about the role and relevance of tumour budding in breast cancers. So, this study attempts to correlate tumour budding, both intra-tumoral and peripheral tumour budding to known clinicopathological prognostic factors of breast carcinoma and hormone receptor status.
Materials and methods
This retrospective observational study was done in the Department of Pathology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal over 2 years from 1st January 2020 to 31st December 2021.
All cases diagnosed as Invasive Breast Carcinoma, no special type (IBC NST) and who underwent surgical resection (Radical or simple mastectomy) in our hospital were included. Patients who underwent core biopsy or lumpectomy, who received any pre-surgical therapy, who were identified to have distant metastasis at the time of primary tumour diagnosis or where clinical data and histopathological slides were unavailable, were excluded from the study. Patients with only core biopsy done were excluded from the study but the ones followed by radical or simple mastectomy were included in the study. Breast conservation surgery specimens were limited in number and were excluded.
The clinical details of these patients such as age, sex, presenting symptoms, details of previous therapy, radiological details and follow-up were retrieved from LIS/RISPACS/EMR discharge summaries and the Medical Records Department. The paraffin blocks and haematoxylin and eosin (H&E) stained slides for the corresponding case numbers of each patient were retrieved from the pathology archives. The gross features were analysed from the pathology reports in the database. The histopathological parameters, hormone receptor status and molecular subtype were analysed from archived pathology data and slides.
Assessment of tumour budding
In this study, we defined tumour budding as an isolated single cancer cell or a cluster of up to 5 tumour cells detached from the main bulk of the tumour, showing no features of differentiation. In this study, we studied tumour budding
• At the invasive front (PTB)
• Inside the body of the tumour (ITB).
For all cases, 2 sections each of the tumour tissue proper and tumour with normal breast interface were studied for assessment of intra-tumoral and peripheral tumour budding respectively. This assessment was performed by both the pathologists, individually and later together to give a consensus value by studying the microscopy on a double-headed microscope. Hot spots were identified by studying the 2 sections each of the tumour proper and tumour with normal breast interface entirely. The intra- and peripheral tumour budding were counted on 20X field in the identified hot spot. This has been illustrated in Figure 1.
FIGURE 1
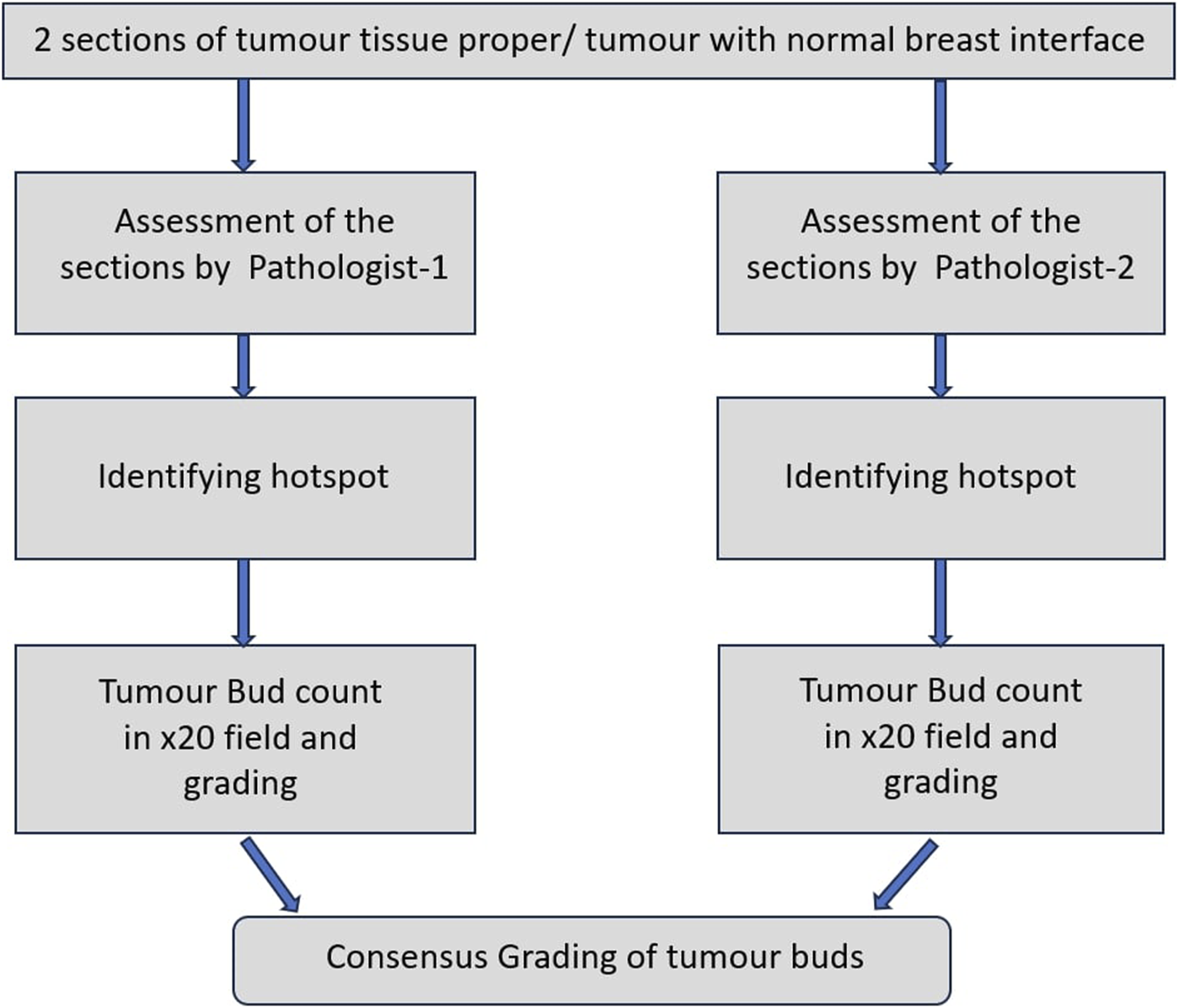
Work-flow diagram.
We separated the cases into 2 categories according to tumour bud density for both ITB and PTB per x20 field (tumour bud density) in hot spot areas [
12].
• Low grade: <10 tumour buds per x20 field
• High grade: ≥10 tumour buds per x20 field
Slide images of low and high grade PTB and ITB are illustrated in
Figures 2–
5.
FIGURE 2
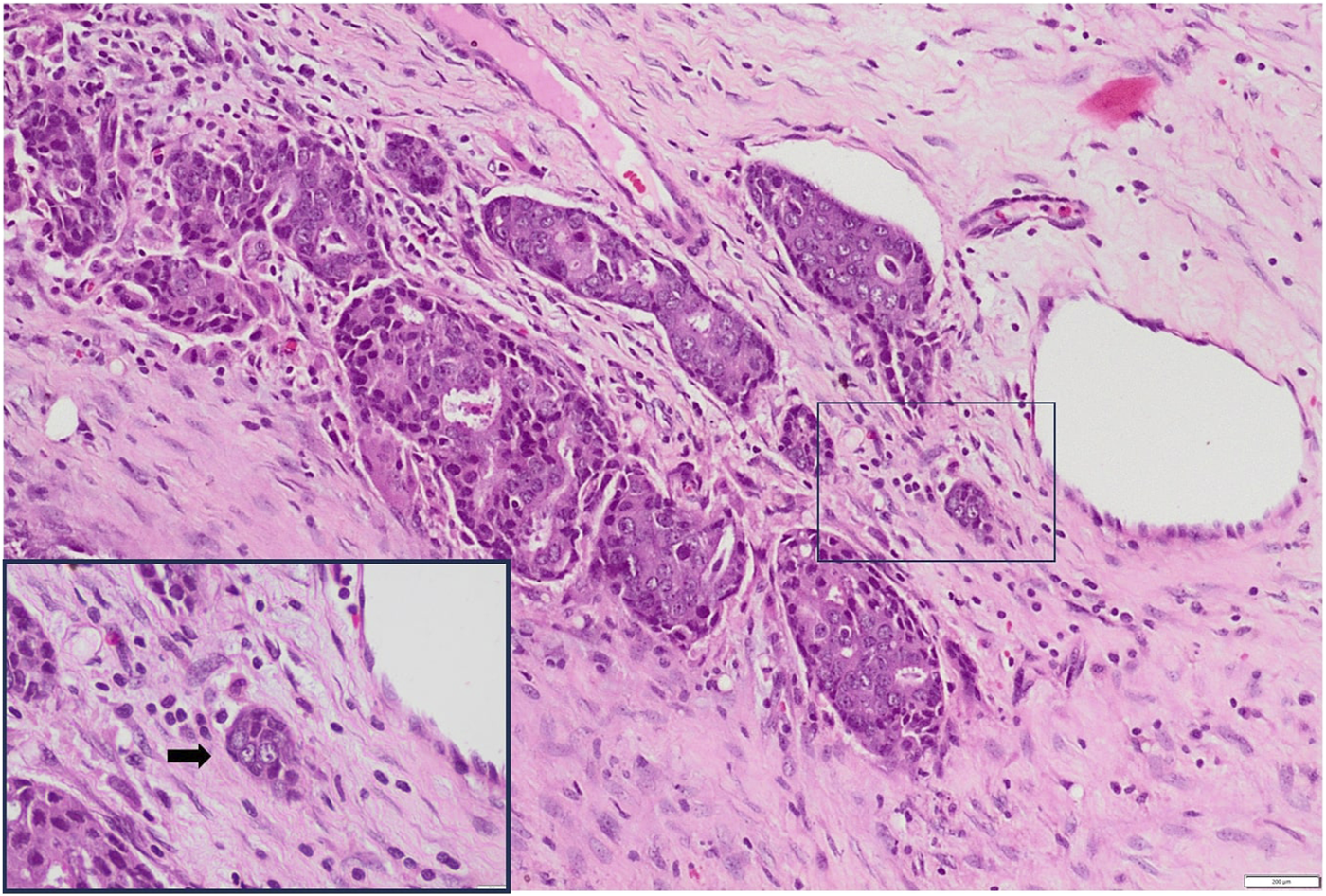
Low-grade PTB H&E X100 (Inset X400).
FIGURE 3
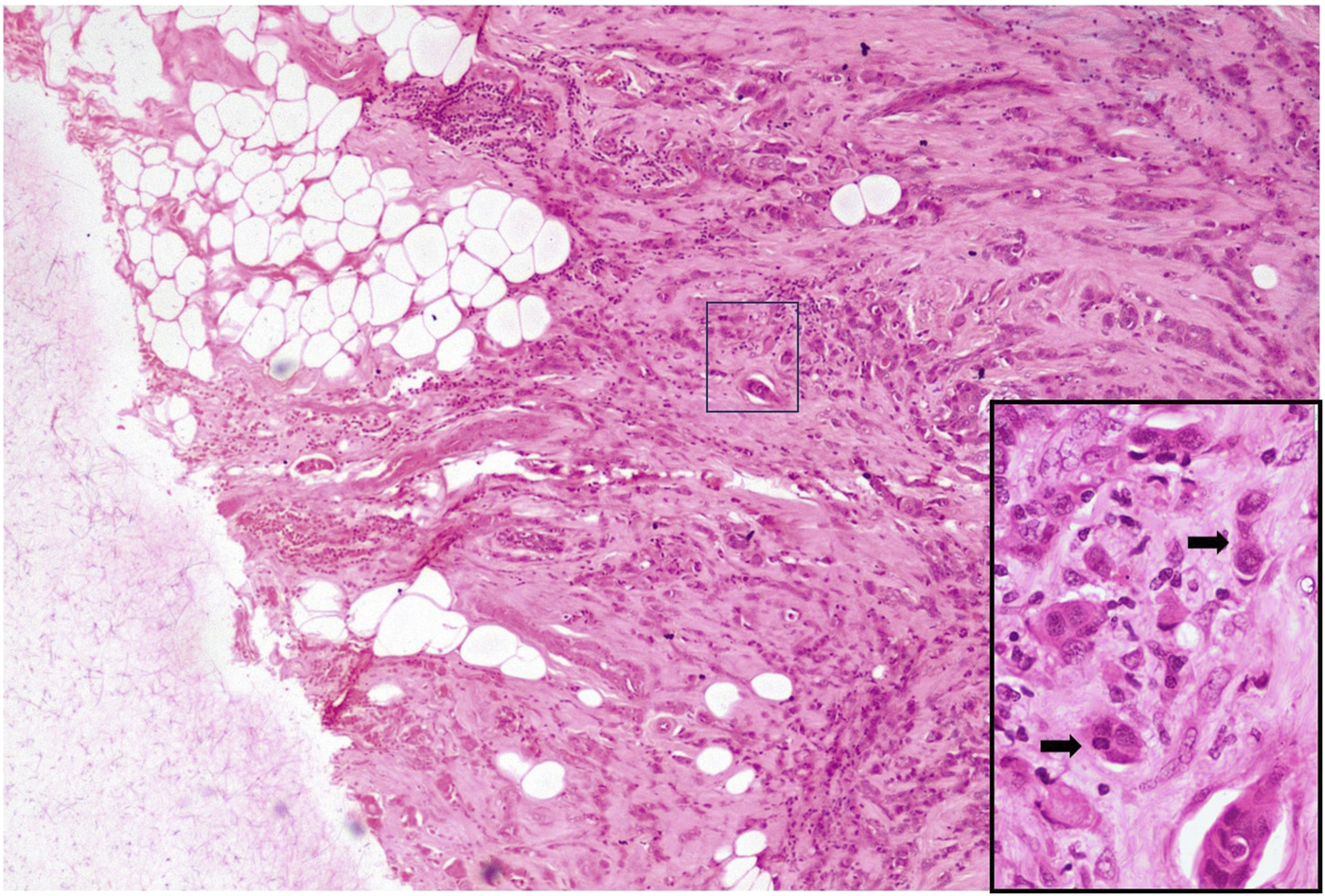
High-grade PTB H&E X40 (Inset X400).
FIGURE 4
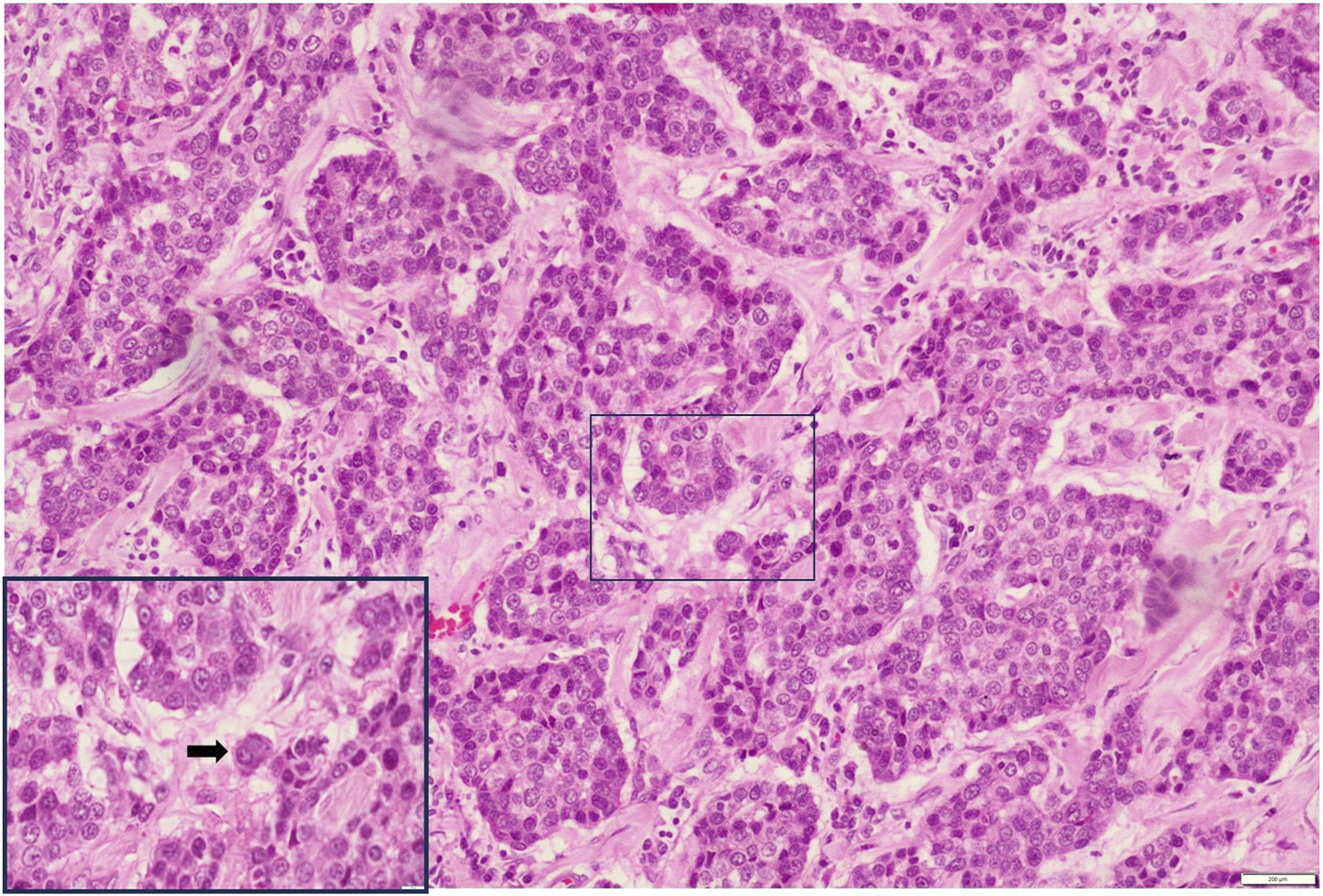
Low-grade ITB H&E X200 (Inset X400).
FIGURE 5
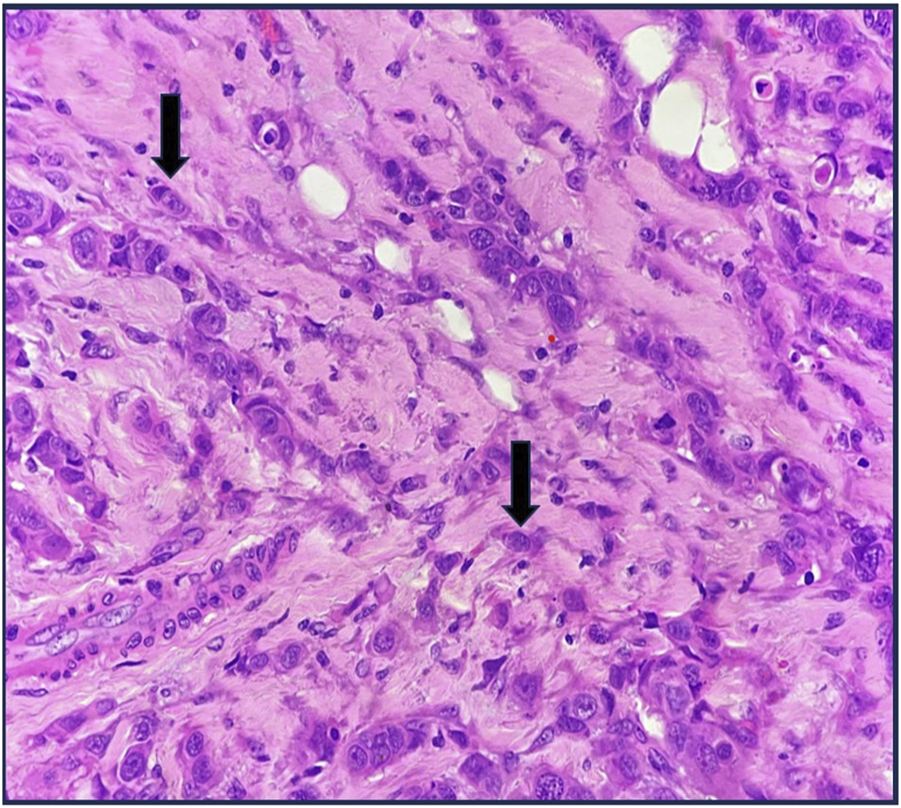
High-grade ITB H&E X400.
Statistical analysis
The collected data were entered into Microsoft Excel 2016 and analysed using IBM SPSS Statistics for Windows, Version 27. The data descriptive statistics were described using frequency analysis, percentage analysis while categorical variables and the median & standard deviation were used for continuous variables. The significance of categorical data was ascertained using the Chi-Square test. The variables which were significant with the Chi-Square test were further analysed by univariate and multivariate logistic regression analyses. In all the above statistical tools a p-value of 0.05 or less is considered as significant level.
Results
In this study, tumour budding in invasive breast carcinomas was studied for a period of 2 years with 70 cases. All the patients in this study were females and had a wide age distribution ranging from 25 to 85 years, with the mean age being 57.23 years. The majority of the patients, 29 (41.4%), were >60 years.
51.4% of the patients had attained menopause while 34 (48.6%) were pre or peri menopausal.
In the vast majority of the cases, 54 (77.2%) had the greatest tumour dimension of 2–5 cm.
Thirty-two cases (45.7%) were graded as Grade 2 according to the Nottingham Grading system, while 28 (40.0%) cases and 10 (14.3%) were graded as Grade 3 and Grade 1, respectively.
Out of 70 cases, only 29 (41.4%) cases were positive for lympho-vascular invasion as 10 (14.2%) cases showed perineural invasion. Five cases showed both LVI and PNI. Positive axillary lymph node metastasis was found in 41 (58.6%) cases.
ER and PR positivity was seen in 52 (74.3%) and 42 (60%) cases respectively. Her2neu was positive in 20 (28.16%) cases.
The majority of the cases showed a high (>20%) Ki-67 index. Table 1 illustrates all the above clinicopathological parameters.
TABLE 1
| Parameter | Frequency (n = 70) |
|---|---|
| Menstrual status: • Pre/Peri- menopausal • Post- menopausal |
34 (48.6%) 36 (51.4%) |
| Tumour Size: • <2 cm • 2–5 cm • >5 cm |
8 (11.4%) 54 (77.2%) 8 (11.4%) |
| Nottingham Grade: • Grade 1 • Grade 2 • Grade 3 |
10 (14.3%) 32 (45.7%) 28 (40.0%) |
| LVI | 29 (41.4%) |
| PNI | 10 (14.3%) |
| Axillary Lymph Node involvement: • Positive • Negative |
41 (58.6%) 29 (41.4%) |
| Hormone Receptors: • ER+ • PR+ • HER2+ |
52 (74.3%) 42 (60%) 20 (28.16%) |
| Ki-67: • 0%–20% • >20% |
12 (17.1%) 58 (82.9%) |
| Molecular Subtype: • Luminal A • Luminal B • HER2 enriched • TNBC |
9 (12.9%) 46 (65.7%) 6 (8.5%) 9 (12.9%) |
| ITB: • Low • High |
46 (65.7%) 24 (34.3%) |
| PTB: • Low • High |
50 (71.4%) 20 (28.6%) |
Distribution of clinicopathological parameters.
Both pre-menopausal and post-menopausal cases predominantly exhibited low-grade intra-tumoral budding (ITB) and peri-tumoral budding (PTB), with a slight increase in high-grade PTB and ITB observed in post-menopausal cases. However, the correlation between tumour budding and menopausal status was not statistically significant. Tumour budding generally remained low-grade across all tumour size groups, though high-grade ITB was present in 83.3% of tumours smaller than 2 cm. Despite this observation, p-values indicated no statistically significant difference in tumour budding distribution across different tumour sizes for ITB and PTB.
Grade 1 tumours showed a high proportion of low-grade ITB and PTB, while high-grade ITB was most prevalent in grade 2 tumours, and high-grade PTB was most common in grade 3 tumours. However, these correlations were not statistically significant. High-grade PTB was significantly associated with lymphovascular (p = 0.046) and perineural invasion (p = 0.017). In contrast, despite being more frequent in cases with LVI and PNI, high-grade ITB did not show a significant correlation. Both high-grade ITB (p = 0.044) and PTB (p = 0.021) were significantly linked to axillary lymph node involvement.
In terms of breast cancer subtypes, high-grade ITB and PTB were more frequently observed in Luminal A type cancers, with the lowest proportions found in triple-negative breast cancers. However, this correlation was not significant (p-values of 0.801 for ITB and 0.183 for PTB). High-grade tumour budding was more common in tumours with low Ki-67 index (0%–20%) for both ITB (41.7%) and PTB (58.3%), with the correlation being significant for PTB (p = 0.012).
LVI (p-value = 0.050), PNI (p-value = 0.025), axillary lymph node involvement (p-value = 0.027), and Ki-67 (p-value = 0.018), index showed significant association with PTB on univariate logistic regression analysis, while only Ki-67 (p-value = 0.017) index showed significant association with PTB on multivariate logistic regression analysis, although PNI (p-value = 0.074) showed near significance. Association of PTB with clinicopathological parameters, along with univariate and multivariate analysis are illustrated in Tables 2, 3.
TABLE 2
| Parameter | PTB | p-value | ||
|---|---|---|---|---|
| Low (n = 50) | High (n = 20) | |||
| Menopausal status | Pre/Peri-Menopausal | 25 (73.5%) | 9 (26.5%) | 0.705 |
| Post- Menopausal | 25 (69.4%) | 11 (30.6%) | ||
| Tumour Size | <2 cm | 4 (50%) | 4 (50%) | 0.361 |
| 2–5 cm | 40 (74%) | 14 (26%) | ||
| >5 cm | 6 (75%) | 2 (25%) | ||
| Nottingham Grade | Grade 1 | 8 (80%) | 2 (20%) | 0.764 |
| Grade 2 | 23 (71.9%) | 9 (28.1%) | ||
| Grade 3 | 19 (67.9%) | 9 (32.1%) | ||
| LVI | Present | 17 (58.6%) | 12 (41.4%) | 0.046 |
| Absent | 33 (80.4%) | 8 (19.6%) | ||
| PNI | Present | 4 (40%) | 6 (60%) | 0.017 |
| Absent | 46 (76.6%) | 14 (23.4%) | ||
| Axillary LN involvement | Present | 25 (61%) | 16 (39%) | 0.021 |
| Absent | 25 (86.2%) | 4 (13.8%) | ||
| Molecular Subtypes | Luminal A | 5 (55.5%) | 4 (44.5%) | 0.183 |
| Luminal B | 30 (65.2%) | 16 (34.7%) | ||
| HER2 Enriched | 4 (66.6%) | 2 (33.3%) | ||
| TNBC | 7 (77.8%) | 2 (22.2%) | ||
| Ki-67 index | 0%–20% | 5 (41.7%) | 7 (58.3%) | 0.012 |
| >20% | 45 (77.6%) | 13 (22.4%) | ||
Correlation of PTB with clinicopathological parameters.
TABLE 3
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Unadjusted OR with 95%CI | p-value | Adjusted OR with 95% CI | p-value | |
| LVI | 2.912 (1.00–8.48) | 0.050 | 2.104 (0.608–7.277) | 0.240 |
| PNI | 4.92 (1.21–19.97) | 0.025 | 3.974 (0.874–18.058) | 0.074 |
| Axillary LN involvement | 4.00 (1.17–13.65) | 0.027 | 2.975 (0.694–12.750) | 0.142 |
| Ki-67 index | 0.206 (0.056–0.759) | 0.018 | 0.169 (0.039–0.725) | 0.017 |
Univariate and multivariate analysis of LVI, PNI, axillary lymph node involvement and Ki-67 with PTB.
OR, Odds ratio.
CI, Confidence interval.
In case of ITB, only axillary lymph node involvement (p-value = 0.048) showed significant association on univariate analysis. Association of ITB with clinicopathological parameters and univariate analysis are illustrated in Tables 4, 5.
TABLE 4
| Parameter | ITB | p-value | ||
|---|---|---|---|---|
| Low (n = 46) | High (n = 24) | |||
| Menopausal status | Pre/Peri-Menopausal | 24 (70.5%) | 10 (29.4%) | 0.404 |
| Post- Menopausal | 22 (61.1%) | 14 (38.9%) | ||
| Tumour Size | <2 cm | 3 (16.7%) | 5 (83.3%) | 0.184 |
| 2–5 cm | 38 (70.3%) | 16 (29.7%) | ||
| >5 cm | 5 (62.5%) | 3 (37.5%) | ||
| Nottingham Grade | Grade 1 | 9 (90%) | 1 (10%) | 0.072 |
| Grade 2 | 17 (53.1%) | 15 (46.9%) | ||
| Grade 3 | 20 (71.4%) | 8 (28.6%) | ||
| LVI | Present | 16 (55.1%) | 13 (44.9%) | 0.118 |
| Absent | 30 (73.1%) | 11 (26.9%) | ||
| PNI | Present | 4 (40%) | 6 (60%) | 0.064 |
| Absent | 42 (70%) | 18 (30%) | ||
| Axillary LN involvement | Present | 23 (56%) | 18 (44%) | 0.044 |
| Absent | 23 (79.3%) | 6 (20.7%) | ||
| Molecular Subtypes | Luminal A | 5 (55.5%) | 4 (44.5%) | 0.801 |
| Luminal B | 30 (65.2%) | 16 (34.7%) | ||
| HER2 Enriched | 4 (66.6%) | 2 (33.3%) | ||
| TNBC | 7 (77.8%) | 2 (22.2%) | ||
| Ki-67 | 0%–20% | 7 (58.3%) | 5 (41.7%) | 0.554 |
| >20% | 39 (67.2%) | 19 (32.8%) | ||
Correlation of ITB with clinicopathological parameters.
TABLE 5
| Parameter | Univariate analysis | |
|---|---|---|
| Unadjusted OR with 95% CI | p-value | |
| LVI | 2.216 (0.810–6.062) | 0.121 |
| PNI | 3.500 (0.880–13.918) | 0.075 |
| Axillary LN involvement | 3.000 (1.009–8.921) | 0.048 |
| Ki-67 | 0.682 (0.191–2.433) | 0.555 |
Univariate analysis of LVI, PNI, axillary lymph node involvement and Ki-67 with ITB.
OR, Odds ratio.
CI, Confidence interval.
Discussion
Breast carcinoma is one of the most prevalent cancers worldwide, contributing significantly to mortality and morbidity. Consequently, extensive research has been conducted to identify prognostic factors associated with the disease, aiming to reduce its impact. This study attempts to correlate tumour budding, which has been established as a prognostic factor in other carcinomas, with known clinicopathological prognostic factors for breast carcinoma.
All patients in this study were female, consistent with the findings of Agarwal et al. [12], Liang et al. [3], and Salhia et al. [15]. However, Silva et al. [16] reported 2% of cases being male and 98% female. In this study, 36 (51.4%) patients were post-menopausal, aligning with the findings of González et al. [17] and Mozarowski et al. [4]. In contrast, Agarwal et al. [12] reported that 55% of patients were pre-menopausal, a difference likely due to the fact that only 14 (35%) of cases in Agarwal’s study involved patients over 50, while in the present study, 29 (41.4%) were over 60 years old.
The tumour size distribution in this study, with a maximum dimension of 2–5 cm, mirrors the findings of Chandana et al. [18], Agarwal et al. [12] and Singh et al. [19] grouped tumours into two categories (≤5 cm and >5 cm) and similarly found that most tumours were 5 cm or smaller. Gujam et al. [20], however, found that most tumours were ≤2 cm, a difference that may be attributed to geographical and economic factors, as the study was conducted in the UK, where greater patient awareness and better access to screening lead to earlier diagnosis.
In this study, most cases were of Nottingham Grade 2 (n = 32, 45.7%), consistent with the findings of Salhia et al. [15] and Chandana et al. [18]. Similarly, Liang et al. [3] and Singh et al. [19] reported approximately 70% of cases as Grade 2. However, studies by Agarwal et al. [12], Muda et al. [21], and Rathod et al. [22] reported a higher proportion of Grade 3 tumours, which could be due to differences in sample size, inter-observer variability in applying grading criteria, temporal variations and population differences.
Lymphovascular invasion (LVI) was found in 29 (41.4%) cases, consistent with the findings of Agarwal et al. [12], Liang et al. [3], Salhia et al. [15], Gupta et al. [23], and Öztürk et al. [24]. However, discordance was noted in the studies by Muda et al. [21], Singh et al. [19], and Kumarguru et al. [25]. Perineural invasion was observed in only a minority of cases, similar to the findings of Salhia et al. [15], Muda et al. [21], and Öztürk et al [24].
Axillary lymph node status is a critical prognostic indicator in breast carcinoma. In this study, 41 (58.6%) cases showed axillary lymph node positivity, consistent with the observations of Agarwal et al. [12], Singh et al. [19], Rathod et al. [22], and Kumarguru et al [25]. Discordance was noted in the studies by Gupta et al. [23] and Chandana et al. [18], which reported lower lymph node positivity, likely due to smaller sample sizes and lower LVI frequency, which is positively associated with lymph node metastasis. However, LVI (p-value = 0.240) and axillary lymph node involvement (p-value = 0.142) did not show any association with PTB on multivariate analysis and PNI (p-value = 0.074) near significant association with PTB. Only axillary lymph node involvement (p-value = 0.048) showed a significant association with ITB on univariate analysis.
The majority of cases in this study were of the Luminal B subtype, similar to Silva et al. [16] findings. However, González et al. [17] and Masilamani et al. [26] reported a higher proportion of Luminal A cases, which could be attributed to variations in sample size, geographical, and ethnic differences in the population studied.
In correlation with patient age, both intra-tumoral budding (ITB) and peri-tumoral budding (PTB) exhibited higher-grade budding in older age groups, which aligns with the findings of Liang et al. [3], Gujam et al. [20], and González et al. [17] However, most studies, including this one, did not find a statistically significant association between tumour budding and age.
In this study, we observed a decrease in the proportion of high-grade PTB as tumour size increased, although this finding was not statistically significant (p = 0.361). This is contrary to the majority of studies, including those by Liang et al. [3], Agarwal et al. [12], Kumaraguru et al. [25], Öztürk et al [24], Silva et al. [16], and Muda et al. [21], which found that high-grade tumour budding was more common in larger tumours, with statistically significant associations. For ITB, a similar pattern of decreasing high-grade budding with increasing tumour size was observed (p = 0.184), while Singh et al. [19] reported increased high-grade budding in both small and large tumours. The discrepancy in findings may be because many cases with tumours smaller than 2 cm had positive axillary lymph node involvement or lymphovascular invasion, which could act as confounding factors.
No significant association between histologic tumour grade and tumour budding was found in either ITB or PTB. In ITB, a higher proportion of high-grade budding was observed in Grade 2 tumours, consistent with the findings of Singh et al. [19] and Salhia et al. [15], although the association in this study was not statistically significant. The lack of significance may be attributed to the smaller sample size. In PTB, high-grade budding increased with tumour grade (p = 0.764), which is in line with the findings of Agarwal et al. [12] and Muda et al. [21], although Liang et al. [3] reported more high-grade budding in Grade 2 tumours. Muda et al. [21] also found a statistically significant association.
This study found a greater proportion of high-grade PTB in Luminal A subtypes, consistent with the findings of Gujam et al. [20] and Masilamani et al. [26], while Öztürk et al. [24] reported more high-grade PTB in Luminal B cases. For ITB, Salhia et al. [15] reported greater high-grade budding in the Luminal A subtype, which is consistent with the findings of this study.
This study also explored the relationship between Ki-67 and tumour budding (both ITB and PTB), revealing a significant inverse association between PTB and Ki-67 (p = 0.012), which persisted in both univariate and multivariate analyses. Ki-67 serves as a marker of tumour proliferative activity, while tumour budding reflects the tumour’s invasive and metastatic potential. Studies on p16(INK4a) and Ki-67 have demonstrated a cessation of proliferative activity at the invasive front of tumours, suggesting that invasion is not synonymous with proliferation. Our findings align with this concept, as the inverse relationship between PTB and Ki-67 indicates a phenotypic shift in tumour cells from a proliferative state to an invasive one [27–29]. Liang et al. [3] had previously validated that budded cells exhibit lower proliferative activity than tumour cells in other areas of the tumour, consistent with studies on tumour budding in colorectal carcinomas.
The strength of this study lies in its comprehensive analysis of both ITB and PTB, examining their relationships with various established prognostic factors—a focus rarely undertaken in previous research. Notably, we demonstrated that PTB is significantly associated with axillary lymph node positivity, LVI, PNI, and Ki-67, while ITB showed a correlation with axillary lymph node positivity. However, in multivariate analysis, only the association between PTB and Ki-67 remained significant.
The retrospective nature and the incorporation of only 70 cases of IBC NST are the limitations of this study as the small sample size limits the generalizability of the findings in this study and the retrospective nature limits the analysis to existing data, which might not have included all variables of interest. This in turn limits the ability to control the confounding factors and biases inherent in the data collection process. As the study was conducted in a single tertiary care centre, the external validity of the results can be in question as the findings might not apply to other populations. Our study mentions the lack of a standard method for quantifying tumour budding. This could have affected the consistency and reliability of the results. We also did not employ any immunohistochemical stains to assist in detecting tumour buds. For this study, we probably excluded some of the clinically relevant subgroups, e.g., post-neoadjuvant therapy cases or cases with breast conservative surgery. According to us, this study primarily focused on histopathological parameters and incorporated only limited follow-up data. Variables like long-term and disease-free survival were not analysed, which could be crucial for understanding the prognostic significance of tumour budding.
Conclusion
This study evaluated tumour budding in terms of peripheral and intra-tumoral budding, categorising them into low-grade and high-grade. Peripheral tumour budding was significantly associated with several poor prognostic factors in invasive breast carcinoma of no special type, including LVI, PNI, and axillary lymph node involvement, however, no significant association was demonstrated in multivariate analysis. In contrast, intra-tumoral budding showed a significant association only with axillary lymph node involvement. Additionally, Ki-67 was seen to be inversely associated with peripheral tumour budding, even on multivariate analysis, suggesting that tumour proliferation and invasion are distinct processes. Given its potential prognostic value, tumour budding could be considered for inclusion in histopathological reporting protocols for breast carcinomas to complement conventional prognostic parameters. Further research is needed to establish a precise definition of tumour budding, including the number of cells forming a bud, the location of budding, standardized quantification methods, and the use of immunohistochemical stains to enhance detection.
Statements
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by IEC2:470/2022, Kasturba Hospital Institutional Ethical Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SF, Concept, collection of material and data, microscopic evaluation, drafting of the manuscript. SS, Concept, microscopic evaluation, critical revision and final drafting of the manuscript.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1.
Siegel RL Miller KD Jemal A . Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. 10.3322/caac.21590
2.
Bray F Laversanne M Sung H Ferlay J Siegel RL Soerjomataram I et al Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2024) 74(3):229–63. 10.3322/caac.21834
3.
Liang F Cao W Wang Y Li L Zhang G Wang Z . The prognostic value of tumor budding in invasive breast cancer. Pathol - Res Pract (2013) 209(5):269–75. 10.1016/j.prp.2013.01.009
4.
Mozarowski P Rasaiah B Reed M Lewis A Walde N Voutsadakis IA . Prognostic role of tumor budding in breast cancer patients receiving neo-adjuvant therapy. J Clin Med (2021) 10(4):827. 10.3390/jcm10040827
5.
Voutsadakis IA . Prognostic role of tumor budding in breast cancer. World J Exp Med (2018) 8(2):12–7. 10.5493/wjem.v8.i2.12
6.
Grigore A Jolly M Jia D Farach-Carson M Levine H . Tumor budding: the name is EMT. Partial EMT. J Clin Med (2016) 5(5):51. 10.3390/jcm5050051
7.
Dawson H Lugli A . Molecular and pathogenetic aspects of tumor budding in colorectal cancer. Front Med (2015) 2:11. 10.3389/fmed.2015.00011
8.
Kalluri R Weinberg RA . The basics of epithelial-mesenchymal transition. J Clin Invest (2009) 119:1420–8. 10.1172/JCI39104
9.
Micalizzi DS Farabaugh SM Ford HL . Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia (2010) 15:117–34. 10.1007/s10911-010-9178-9
10.
Nieto MA . Epithelial plasticity: a common theme in embryonic and cancer cells. Science (2013) 342:1234850. 10.1126/science.1234850
11.
Imai T . Growth patterns in human carcinoma. Their classification and relation to prognosis. Obstet Gynecol (1960) 16:296–308.
12.
Agarwal R Khurana N Singh T Agarwal P . Tumor budding in infiltrating breast carcinoma: correlation with known clinicopathological parameters and hormone receptor status. Indian J Pathol Microbiol (2019) 62(2):222–5. 10.4103/IJPM.IJPM_120_18
13.
Li W Lu N Chen C Lu X Li W Lu N et al Identifying the optimal cutoff point of Ki-67 in breast cancer: a single-center experience. J Int Med Res (2023) 51(8):3000605231195468. 10.1177/03000605231195468
14.
Bustreo S Osella-Abate S Cassoni P Donadio M Airoldi M Pedani F et al Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: a large case series study with a long-term follow-up. Breast Cancer Res Treat (2016) 157:363–71. 10.1007/s10549-016-3817-9
15.
Salhia B Trippel M Pfaltz K Cihoric N Grogg A Lädrach C et al High tumor budding stratifies breast cancer with metastatic properties. Breast Cancer Res Treat (2015) 150(2):363–71. 10.1007/s10549-015-3333-3
16.
Silva DJ Miranda G Amaro T Salgado M Mesquita A . Prognostic value of tumor budding for early breast cancer. Biomedicines (2023) 11(11):2906. 10.3390/biomedicines11112906
17.
González LO Eiro N Fraile M Sánchez R Andicoechea A Fernández-Francos S et al Joint tumor bud–MMP/TIMP count at the invasive front improves the prognostic evaluation of invasive breast carcinoma. Biomedicines (2021) 9(2):196. 10.3390/biomedicines9020196
18.
K A . The role of tumour budding as a prognostic factor in primary invasive ductal carcinoma of breast. Online J Health Allied Sci (2022) 21(3), 9. Available from: https://www.ojhas.org/issue83/2022-3-9.html (Accessed January 22, 2024).
19.
Singh T Chandra K Kumar N Mishra A Singh S Singh A et al A retrospective study of association of tumor budding, tumor microenvironment, and clinicopathological characteristics of invasive breast carcinoma. J Lab Physicians (2022) 14(04):485–90. 10.1055/s-0042-1747676
20.
Gujam FJA McMillan DC Mohammed ZMA Edwards J Going JJ . The relationship between tumour budding, the tumour microenvironment and survival in patients with invasive ductal breast cancer. Br J Cancer (2015) 113(7):1066–74. 10.1038/bjc.2015.287
21.
Muda NM Deshmukh AV Shivkumar VB Shirbhate PN . Role of tumour budding in breast cancer and its correlation with other prognostic factors: a cross sectional study in rural Central India. Indian J Gynecol Oncol (2024) 22(1):6. 10.1007/s40944-023-00769-7
22.
Rathod GB Desai KN Shrivastava A Maru AM . Correlation of tumor budding with known clinicopathological, histomorphological and hormonal receptor status in patients with invasive breast carcinoma. Cureus (2022) 14(9):e29637. 10.7759/cureus.29637
23.
Gupta V Patel M . Evaluation of prognostic role of tumour budding in breast carcinoma and its correlation with known clinicopathological parameters. Indian J Pathol Oncol (2022) 9(3):238–42. 10.18231/j.ijpo.2022.056
24.
Öztürk Ç Aşkan G Öztürk SD Okcu O Şen B Bedir R . Does the number of cell forming tumor budding alter the prognostic value in invasive ductal carcinoma of breast?Pathol - Res Pract (2022) 240:154157. 10.1016/j.prp.2022.154157
25.
Kumarguru B Ramaswamy A Shaik S Karri A Srinivas V Prashant B . Tumor budding in invasive breast cancer - an indispensable budding touchstone. Indian J Pathol Microbiol (2020) 63(5):117–S122. 10.4103/IJPM.IJPM_731_18
26.
Masilamani DS P DK . Evaluation of clinicopathologic significance of tumor budding in breast carcinoma. Int J Clin Diagn Pathol (2019) 2(1):171–3. 10.33545/pathol.2019.v2.i1c.25
27.
Svensson S Nilsson K Ringberg A Landberg G . Invade or proliferate? Two contrasting events in malignant behavior governed by p16(INK4a) and an intact Rb pathway illustrated by a model system of basal cell carcinoma. Cancer Res (2003) 63(8):1737–42.
28.
Jie G Zhixiang S Lei S Hesheng L Xiaojun T . Relationship between expression and methylation status of p16INK4a and the proliferative activity of different areas’ tumour cells in human colorectal cancer: p16INK4a gene and the proliferative activity of different areas’ tumour cells in colorectal cancer. Int J Clin Pract (2007) 61(9):1523–9. 10.1111/j.1742-1241.2006.01033.x
29.
Kin R Hoshi D Fujita H Kosaka T Takamura H Kiyokawa E . Prognostic significance of p16, p21, and Ki67 expression at the invasive front of colorectal cancers. Pathol Int (2023) 73(2):81–90. 10.1111/pin.13295
Summary
Keywords
invasive breast carcinoma, intra tumoral budding, peripheral tumour budding, prognostic parameters, Ki-67
Citation
Francis SS and Sharma S (2025) Tumour budding in invasive ductal breast carcinomas: correlation with clinicopathological prognostic parameters and hormone receptor status. Pathol. Oncol. Res. 31:1611983. doi: 10.3389/pore.2025.1611983
Received
30 September 2024
Accepted
27 January 2025
Published
12 February 2025
Volume
31 - 2025
Edited by
Gabor Cserni, University of Szeged, Hungary
Updates
Copyright
© 2025 Francis and Sharma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Swati Sharma, swati.sharma@manipal.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.