Abstract
Background and Objectives:
This study aims to evaluate the correlation between Tumor-Infiltrating Lymphocyte (TIL) levels and Fluorine-18 fluorodeoxyglucose (18F-FDG) metabolic parameters, including spleen and bone marrow FDG uptake and tumor heterogeneity in non-luminal breast cancers (NLBC), and to elucidate their association with survival outcomes.
Methods:
We retrospectively analyzed data from 100 females with stage 2–4 NLBC who underwent pretreatment 18F-FDG Positron emission tomography-computed tomography (PET/CT). TIL was scored based on Hematoxylin-Eosin-stained specimens and 18F-FDG PET metabolic parameters, including maximum standardized uptake value (SUVmax), mean standardized uptake value (SUVmean), metabolic tumor volume (MTV), total lesion glycolysis (TLG), liver, spleen, and bone marrow FDG uptake were calculated. Heterogeneity Index (HI)1, HI2, and HI3 indices were analyzed with FDG metabolic parameters. The association between these factors and overall survival was analyzed using multivariate Cox regression models.
Results:
TIL showed weak negative correlations with tumor size, tumor (T), and metastasis (M) stages. No significant correlation was found between TIL levels and overall SUV values. However, in stage 4, TIL correlated positively with liver, spleen, and bone marrow SUV values and negatively with heterogeneity indices (HI2, HI3). Higher tumor size, HI values, and Bone marrow-to-liver ratio (BLR) SUVmean were associated with increased mortality. A TIL cut-off value of <5 was linked to significantly worse survival.
Conclusion:
Our study demonstrates a strong connection between TIL, FDG metabolic parameters, and tumor heterogeneity, particularly in advanced NLBC. Although TIL is not generally associated with SUV values, its association with certain metabolic and heterogeneity indices suggests that it is important in influencing survival. Further research involving larger cohorts and diverse breast cancer subtypes is needed to validate these results.
Introduction
Non-luminal breast cancers (NLBC), including human epidermal growth factor receptor-2 (HER2) positive and triple-negative breast cancers (TNBC), are characterized by aggressive clinical behavior and poor clinical outcomes [1, 2]. Effective management necessitates a thorough understanding of tumor biology and the interaction of various prognostic variables [3].
Tumor-infiltrating lymphocytes (TILs), immune cells seen in the tumor microenvironment, have been identified as important prognostic indicators in non-luminal breast cancers [1, 4, 5]. Several clinical trials have reported the prognostic and predictive importance of TILs in breast cancer. High TIL levels often indicate a robust immune response, which has been linked to a better prognosis and improved treatment outcomes [3, 6]. Higher Tumor-Infiltrating Lymphocytes have been associated with a better prognosis in both HER2-positive and TNBC patients, indicating a more favorable immunological milieu that may increase responses to targeted therapies and correspond with better clinical outcomes and survival rates [7, 8]. Despite these associations, the variations in clinical outcomes based on differing TIL levels still need to be explored [9].
In addition to TIL, positron emission tomography-computed tomography (PET/CT) imaging of fluorine-18 fluorodeoxyglucose (18F-FDG) metabolic parameters provides useful information on the tumor’s metabolic activity [10–12]. FDG uptake in the spleen and bone marrow on PET/CT is also being recognized as a good biomarker of systemic immune response and tumor-related metabolic alterations [11, 13, 14]. These measures represent the tumor’s level and extent of glucose metabolism, quantifying its aggressiveness and metabolic burden [15].
Another critical factor, tumor heterogeneity, adds complexity to the management of breast cancer [16]. Heterogeneity can manifest in genetic, phenotypic, and microenvironmental variations, influencing tumor behavior and response to treatment [6, 16–18]. Integrating FDG metabolic parameters with tumor heterogeneity assessments can reveal metabolic variability patterns crucial for understanding the tumor’s complexity and tailoring treatment strategies [8]. Tumor-infiltrating lymphocytes play a significant role in the immune response against breast cancer, influencing the tumor microenvironment and heterogeneity.
Understanding the relationships between TIL levels, FDG metabolic parameters, and tumor heterogeneity is essential for refining prognostic assessments and guiding personalized treatment strategies in non-luminal breast cancer [10, 12]. Evaluating the correlation between TIL levels and FDG metabolic parameters could improve predictions of overall survival [19].
This study aims to evaluate the correlation between TIL levels and FDG metabolic parameters, including spleen and bone marrow uptake and tumor heterogeneity, in nonluminal breast cancers and examine their association with survival outcomes.
Methods
Study design and patients’ selection
The records of 100 females with histopathologically confirmed newly diagnosed stage 2–4 nonluminal breast cancer who received pretreatment 18F-FDG PET/CT between June 2016 and September 2023 were reviewed retrospectively. Patients were assessed for pretreatment staging before beginning oncological or surgical treatment. All patients were initially diagnosed with core needle tru-cut biopsy. The study excluded patients with bilateral breast cancer, non-invasive ductal cancer histopathology, secondary primary malignancy diagnosis, pregnancy, male patients, stage 1 disease, insufficient clinical data, immunohistochemical data, no TIL rate, and no follow-up. Following the application of the exclusion criteria, the remaining patients were included in the research.
The patients were staged using the 8th edition of the American Joint Committee on Cancer (AJCC) Tumor-Node-Metastasis (TNM) system, which provides a complete classification for breast cancer based on tumor size (T), lymph node involvement (N), and the existence of distant metastasis (M) [20]. Tumor staging was evaluated using MR and PET/CT scans. The nodal staging was determined by analyzing the imaging data, physical exam, and sentinel lymph node biopsy results. Since staging with 18F-FDG PET/CT has no additional value in clinical stage I (T1N0) patients, the group of nonluminal breast cancer patients with stages 2–4 was included in the study.
Patient data was obtained from patient files, the hospital information management system, and Picture Archive and Communication Systems (PACS). Overall survival (OS) was determined by calculating the months between the date of diagnosis and the date of death registered in the death notification system (DNS). All patients underwent at least 1 year of follow-up.
Pathological analysis
Nonluminal breast cancer includes the HER2-positive group, which is marked by high HER2 expression and the absence of ER and PR, and triple-negative breast cancer, which lacks expression of ER, PR, and HER2 [2].
The immunohistochemistry (IHC) for HER2 was performed using HER2 neu (EP3) monoclonal antibody. HER2 status was determined based on recommendations by The American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines [21]. Tumors were classified according to this guideline as positive (score:+3), equivocal (score:+2), and negative (score:+1/0). The equivocal category was tested by silver in situ hybridization (SISH) to determine whether there was overexpression of HER2 protein. The Ki-67 proliferation rate was counted in hotspot areas, with the percentage of tumor cells showing nuclear Ki-67 positivity being reported.
TIL was scored using the standardized method Products by the International Immuno-Oncology Biomarker Working Group on Breast Cancer [3]. TIL is defined as mononuclear cells that infiltrate tumor tissue. The scoring of TILs was based on Hematoxylin-Eosin (H-E) stained specimens and described as a percentage of the area occupied by TIL per total stromal area. The TIL score of the breast cancers was classified as low (<10%), intermediate (10%–40%), and high (>40%) by a pathologist blinded to the remaining clinical information. Only the index tumor was analyzed in this study if patients had multiple tumors.
18F-FDG PET/CT image technique
All patients underwent PET/CT after fasting for at least 6 h and then had their blood glucose levels checked. A serum glucose level was measured below 200 ng/dL, and an intravenous injection of 8–12 mCi (296–444 MBq) (approximately 8.1 MBq of FDG per kilogram of body weight) 18F-FDG was administered. Whole-body PET/CT imaging was performed on a biograph (Siemens Biograph 6, Chicago, IL, United States) using a full-ring high-resolution (HI-REZ) LSO PET and a six-slice CT scanner (Siemens Biograph 6, Chicago, IL, United States). Firstly, a non-enhanced CT scan was performed with the following parameters: 40–60 mAs, 140 kV, and 5-mm section thickness. Positron emission tomography scanning with 3 min per bed position was then acquired on the identical transverse field of view in the caudocranial direction. PET image datasets were reconstructed iteratively using the ordered subset expectation maximization algorithm with CT-based attenuation correction.
18F-FDG PET/CT image analysis
A General Electric Advantage Workstation (AW workstation Volume Viewer 3 software, version 3.2 (AWS) GE Healthcare, Waukesha, WI, United States) was used for all image analysis. The program was used to segment lesions automatically. Images were localized by starting evaluation with maximum intensity projection (MIP) images. Pre-segmentation of the lesions was performed by two Nuclear Medicine physicians. The lesions were marked with the help of auto-contour ROI (auto-contour ROI), and measurements were performed in all three planes (coronal-axial-sagittal) after it was determined that the entire lesion was within the ROI and that there were no other foci showing FDG uptake outside the lesion.
The tumor’s maximum standardized uptake value (SUVmax), mean standardized uptake value (SUVmean), peak standardized uptake values (SUVpeak), Metabolic Tumor Volume (MTV), and Total Lesion Glycolysis (TLG) were noted. Using the formula [injected dose (MBq) body weight (g)], SUVmax was determined based on body weight. The tumor contours were semi-automatically identified to determine MTV by employing a threshold of 42% of the SUVmax within the lesion. The SUVmean was multiplied by MTV to compute TLG. A volumetric zone of interest was defined to encompass the primary tumor completely. On checking the sagittal and coronal images, the volumetric region of interest border was semi-automatically adjusted if the volume extended beyond the borders of the primary lesion.
The SUVmax and SUVmean values for the liver, spleen, and bone marrow were calculated. Bone marrow SUV measurements were taken from the L2-L4 vertebrae. Subsequently, the following ratios were calculated: the mean SUV of the spleen to the mean SUV of the liver (SLR SUVmean), the SUVmax of the spleen to the SUVmax of the liver (SLR SUVmax), the SUVmax of the bone marrow to the SUVmax of the liver (BLR SUV max), and the mean SUV of the bone marrow to the mean SUV of the liver (BLR SUV mean).
Heterogeneity Index (HI) values and other continuous variables were analyzed: the lesions’ highest standardized uptake values (SUVmax) and peak standardized uptake values (SUVpeak) were recorded. The mean SUV (SUVmean) and minimum SUV (SUVmin) values at the threshold value of 30% were determined over SUVmax. Based on these values, the HI1 value was calculated using the formula (SUVmax-SUVmin)/SUVmean [16].
To calculate other HI values, metabolic tumor volume (MTV) and total lesion glycolysis (TLG) values were determined over the 40% threshold value of SUVmax. Then, the slope of the linear regression line of the MTV values at the threshold values of 30%–40%-50% over the new MTV values obtained by changing the threshold percentage to 30% and 50% values were calculated as HI2 [17].
With the same technique, the slope of the linear regression line obtained with the percentage change of MTV at 40% and 50% threshold values by accepting the MTV at 30% threshold value as 100% was determined as HI3 [18].
Statistical analysis
Statistical Package for Social Sciences version 25.0 (SPSS, IBM Corp. Armonk, NY, USA) for Windows was used for statistical analysis. Descriptive statistics were presented as counts and percentages for categorical variables and mean, standard deviation, minimum, maximum, and median for numerical variables. The proportions between groups were compared using the Chi-square test. Comparisons of numerical variables between groups were performed using the Student's t-test to see if the normal distribution condition was met or the Mann-Whitney U test to see if it was not. Relationships between numerical variables were examined using Spearman Correlation Analysis, as the parametric test conditions were unmet. Determinant factors were analyzed using Linear Regression Analysis and Cox Regression Analysis. Findings that were statistically significant in univariable analysis were included in the multivariable backward model. Receiver operating characteristic (ROC) Curve Analysis was performed for the TIL cut-off value. Survival analyses were conducted using Kaplan-Meier Analysis. The alpha significance level was accepted as p < 0.05.
Ethical approval: The research was authorized by the decision of the Ministry of Health Istanbul, Türkiye Prof. Dr. Cemil Taşcıoğlu City Hospital Local Ethics Committee, numbered 254, dated 07.11.2023 (2023/254). The study was conducted according to the ethical principles in the Declaration of Helsinki and the guidelines of Good Clinical Practice/Good Laboratory Practice standards.
Results
The study included one hundred women with breast cancer. The patients’ mean/median age was 50.48 ± 11.54 (range 27–86). The HER2-positive BC group comprised 39 patients, while TNBC included 61. According to staging data, the disease was in stages 2 for 40 patients, 3 for 35, and 4 for 24 people. Two patients were in T1, 68 in T2, 20 in T3, and 10 in T4 tumoral stages, while 26 were in N0, 26 in N1, 19 in N2, and 29 in N3 nodal staging groups. Twenty-four patients had metastatic disease. The patient characteristics are summarized in Table 1. Of the 100 breast cancer lesions, 11 (11%) were classified as high TIL, 27 (27%) as intermediate TIL, and 62 (62%) as low TIL (Figures 1, 2). TIL had a negative and statistically significant association with tumor size and metastasis (p = 0.016 p = 0.047 p = 0.023) (Table 2). There was no statistical association between TIL levels and SUV values in non-luminal breast cancer.
TABLE 1
| Total | Alive | Exitus | Pa | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| TNBC/HER2 | HER2 | 39 | 39.0 | 33 | 44.0 | 6 | 24.0 | 0.076 |
| TNBC | 61 | 61.0 | 42 | 56.0 | 19 | 76.0 | ||
| Breast side | R | 52 | 52.0 | 37 | 49.3 | 15 | 60.0 | 0.355 |
| L | 48 | 48.0 | 38 | 50.7 | 10 | 40.0 | ||
| Pathologic Diagnosis | IC | 5 | 5.0 | 3 | 4 | 2 | 8.0 | 0.455 |
| IDC | 95 | 95.0 | 72 | 96.0 | 23 | 92.0 | ||
| Nuclear Grade | 2 | 5 | 5.0 | 4 | 5.3 | 1 | 4.0 | 1.000 |
| 3 | 95 | 95.0 | 71 | 94.7 | 24 | 96.0 | ||
| TILs | 0–10 | 62 | 62.0 | 43 | 57.3 | 19 | 76.0 | 0.145 |
| 10–40 | 27 | 27.0 | 24 | 32.0 | 3 | 12.0 | ||
| >40 | 11 | 11.0 | 8 | 10.7 | 3 | 12.0 | ||
| T | 1 | 2 | 2.0 | 2 | 2.7 | 0 | 0.0 | 0.033 |
| 2 | 68 | 68.0 | 56 | 74.7 | 12 | 48.0 | ||
| 3 | 20 | 20.0 | 12 | 16.0 | 8 | 32.0 | ||
| 4 | 10 | 10.0 | 5 | 6.7 | 5 | 20.0 | ||
| N | 0 | 26 | 26.0 | 23 | 30.7 | 3 | 12.0 | 0.151 |
| 1 | 26 | 26.0 | 19 | 25.3 | 7 | 28.0 | ||
| 2 | 19 | 19.0 | 15 | 20.0 | 4 | 16.0 | ||
| 3 | 29 | 29.0 | 18 | 24.0 | 11 | 44.0 | ||
| M | 0 | 76 | 76.0 | 66 | 88.0 | 10 | 44.0 | <0.001 |
| 1 | 24 | 24.0 | 9 | 12.0 | 15 | 56.0 | ||
| Stage | 2A | 25 | 25.0 | 24 | 32.0 | 1 | 4.0 | <0.001 |
| 2B | 16 | 16.0 | 11 | 14.6 | 5 | 16.0 | ||
| 3A | 14 | 14.0 | 14 | 20.0 | 0 | 0.0 | ||
| 3B | 4 | 4.0 | 3 | 6.0 | 1 | 4.0 | ||
| 3C | 17 | 17.0 | 14 | 18.7 | 3 | 12.0 | ||
| 4 | 24 | 24.0 | 9 | 3.7 | 15 | 62.3 | ||
| Multicentricity | 30 | 30.0 | 22 | 29.3 | 8 | 32.0 | 0.801 | |
| Axillary Lymph Node positive | 74 | 74.0 | 52 | 64.0 | 22 | 80.0 | 0.137 | |
| >Follow-up Median (Min-Max) | 34 (9–97) | 39 (10–97) | 21 (9–76) | <0.001 b | ||||
Patient characteristics.
Chi Square Test.
Mann Whitney U Test HER2, Human epidermal growth factor receptor-2; TNBC, triple-negative breast cancer; TILs, tumor-infiltrating lymphocytes; T, tumor; N, lymph node; M, metastasis.
FIGURE 1
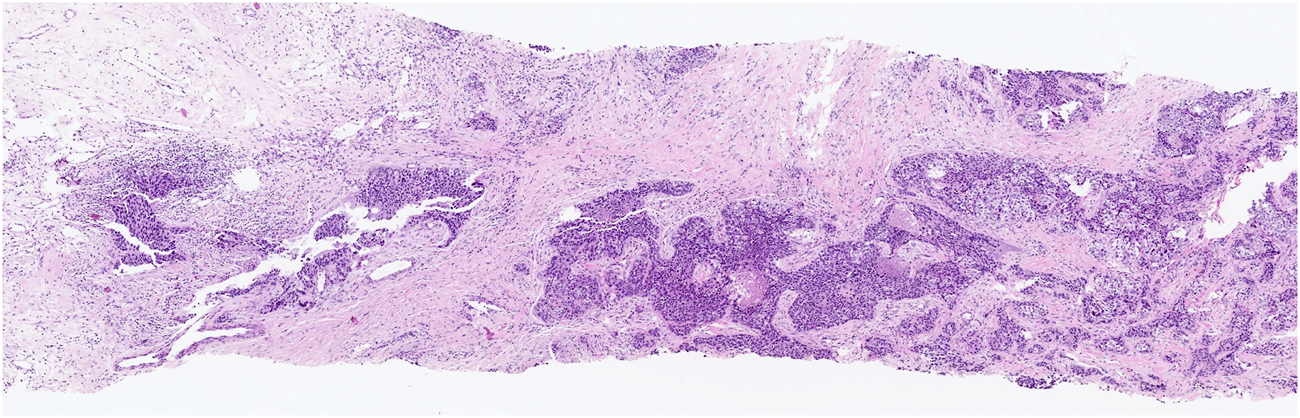
Low-grade TILs: a few lymphocytes in stromal tissue surrounding the cancer.
FIGURE 2
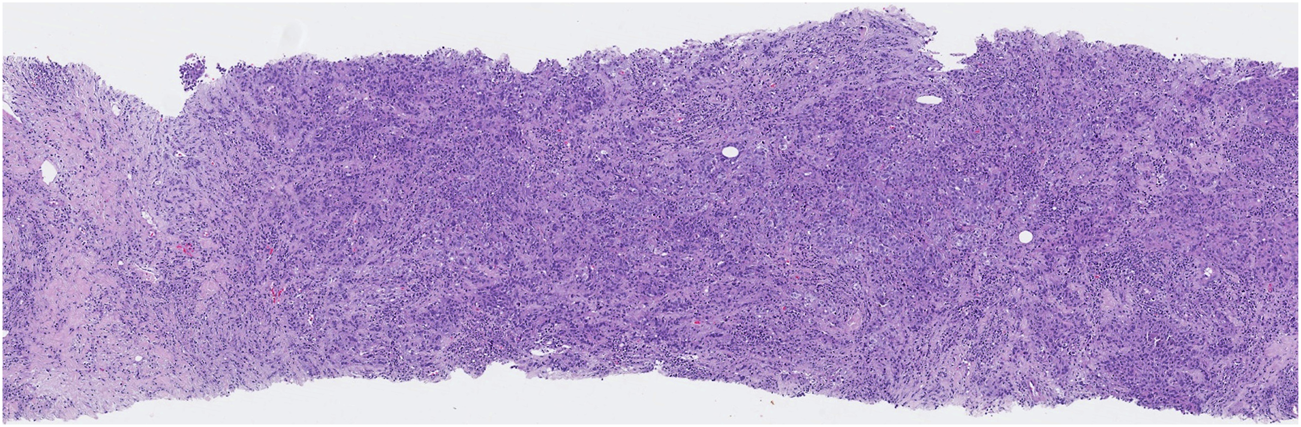
High-grade TILs: numerous lymphocytes are present in stromal tissue adjacent to the cancer.
TABLE 2
| TILs | ||
|---|---|---|
| r | pa | |
| Ki67 | 0.141 | 0.161 |
| Diameter | −0.241 | 0.016 |
| T | −0.199 | 0.047 |
| N | −0.100 | 0.322 |
| M | −0.228 | 0.023 |
| Stage | −0.147 | 0.145 |
| SUVmax | 0.037 | 0.717 |
| SUVmin | −0.014 | 0.891 |
| SUVmean | 0.047 | 0.640 |
| HI1 (max-min/mean) | −0.042 | 0.682 |
| MTV %42 | −0.154 | 0.125 |
| TLG | −0.116 | 0.251 |
| SUVpeak | 0.039 | 0.697 |
| HI2 (MTV threshold index) | −0.192 | 0.056 |
| HI3 (% HI2) | −0.194 | 0.054 |
| Liver SUVmax | 0.061 | 0.545 |
| Liver SUVmean | 0.111 | 0.271 |
| Spleen SUVmax | 0.132 | 0.189 |
| Spleen SUVmean | 0.162 | 0.108 |
| BM SUVmax | 0.072 | 0.479 |
| BM SUVmean | 0.142 | 0.158 |
| SLR SUVmax | 0.017 | 0.867 |
| SLR SUVmean | 0.041 | 0.686 |
| BLR SUVmax | −0.009 | 0.927 |
| BLR SUVmean | 0.082 | 0.418 |
Correlation between TIL and all other parameters in all groups.
Spearman Correlation Analysis.
TILs, tumor-infiltrating lymphocytes; T, Tumor; N, lymph node; M, Metastasis; SUVmax, maximum standardized uptake value; SUVmin, minimum standardized uptake value; SUVmean, mean standardized uptake value; HI, Heterogeneity Index; MTV, metabolic tumor volume; TLG, total lesion glycolysis; SUVpeak, peak standardized uptake value; SLR, spleen-to-liver ratio; BLR, bone marrow-to-liver ratio.
TIL was observed to have a positive correlation (p = 0.027 p = 0.046) with both Liver SUVmax and SUVmean in stage 3 patients. In stage 4, TIL levels were found to have a negative, statistically significant correlation with HI2 and HI3 and a positive, statistically significant correlation with Liver SUVmean, Spleen SUVmax, SUVmean, and Bone Marrow SUVmax, SUVmean (p = 0.023, p = 0.004, p = 0.049, p = 0.004, p = 0.004, p = 0.007, p = 0.009) (Table 3).
TABLE 3
| Stage 2 | Stage 3 | Stage 4 | ||||
|---|---|---|---|---|---|---|
| TILs | TILs | TILs | ||||
| r | p | r | p | r | p | |
| Ki67 | 0.194 | 0.229 | 0.187 | 0.275 | 0.173 | 0.419 |
| Diameter | −0.348 | 0.028 | 0.015 | 0.932 | −0.364 | 0.081 |
| T | −0.333 | 0.035 | 0.012 | 0.946 | −0.231 | 0.278 |
| N | 0.063 | 0.700 | −0.165 | 0.336 | −0.360 | 0.084 |
| M | −0.182 | 0.288 | −0.210 | 0.324 | ||
| SUVmax | 0.012 | 0.940 | 0.046 | 0.788 | 0.097 | 0.653 |
| SUVmin | −0.056 | 0.731 | 0.072 | 0.677 | −0.060 | 0.780 |
| SUVmean | 0.016 | 0.923 | −0.010 | 0.956 | 0.178 | 0.405 |
| HI1(max-min/mean) | −0.091 | 0.575 | 0.043 | 0.803 | 0.023 | 0.915 |
| MTV %42 | −0.055 | 0.735 | 0.050 | 0.772 | −0.404 | 0.050 |
| TLG | −0.071 | 0.664 | 0.037 | 0.830 | −0.206 | 0.334 |
| SUVpeak | −0.014 | 0.934 | 0.089 | 0.607 | 0.166 | 0.439 |
| HI2 | −0.152 | 0.350 | 0.047 | 0.786 | −0.462 | 0.023 |
| HI3 (% HI2) | −0.052 | 0.751 | −0.090 | 0.602 | −0.563 | 0.004 |
| Liver SUVmax | −0.261 | 0.104 | 0.367 | 0.027 | 0.270 | 0.202 |
| Liver SUVmean | −0.161 | 0.322 | 0.335 | 0.046 | 0.406 | 0.049 |
| Spleen SUVmax | −0.177 | 0.276 | 0.277 | 0.102 | 0.567 | 0.004 |
| Spleen SUVmean | −0.139 | 0.393 | 0.311 | 0.064 | 0.560 | 0.004 |
| BM SUVmax | −0.222 | 0.169 | 0.090 | 0.602 | 0.539 | 0.007 |
| BM SUVmean | −0.119 | 0.466 | 0.236 | 0.167 | 0.523 | 0.009 |
| SLR SUVmax | −0.034 | 0.837 | 0.090 | 0.602 | 0.038 | 0.860 |
| SLR SUVmean | −0.048 | 0.771 | 0.113 | 0.511 | 0.149 | 0.487 |
| BLR SUVmax | −0.077 | 0.637 | −0.116 | 0.501 | 0.242 | 0.254 |
| BLR SUVmean | −0.023 | 0.889 | 0.099 | 0.564 | 0.328 | 0.118 |
Correlation between TIL and Stages with all other parameters.
Spearman Correlation Analysis.
TILs, tumor-infiltrating lymphocytes; T, tumor; N, lymph node; M, Metastasis; SUVmax, maximum standardized uptake value; SUVmin, minimum standardized uptake value; SUVmean, mean standardized uptake value; HI, Heterogeneity Index; MTV, metabolic tumor volume; TLG, total lesion glycolysis; SUVpeak, peak standardized uptake value; SLR, spleen-to-liver ratio; BLR, bone marrow-to-liver ratio.
TIL correlated positively with Ki67 at low levels, negatively with MTV%42, and adversely with metastasis in HER2-positive patients (p = 0.015, p = 0.045, p < 0.001). In TNBC patients, low TIL levels were observed to be inversely linked with tumor growth, HI1, and HI3 (Table 4).
TABLE 4
| HER2 | TNBC | |||
|---|---|---|---|---|
| TILs | TILs | |||
| r | p | r | p | |
| Ki67 | 0.388 | 0.015 | −0.042 | 0.747 |
| Diameter | −0.239 | 0.143 | −0.256 | 0.046 |
| T | −0.264 | 0.104 | −0.217 | 0.093 |
| N | −0.192 | 0.240 | −0.043 | 0.744 |
| M | −0.560 | <0.001 | −0.084 | 0.520 |
| Stage | −0.277 | 0.088 | −0.061 | 0.639 |
| SUVmax | 0.000 | 1.000 | −0.004 | 0.977 |
| SUVmin | −0.035 | 0.830 | 0.015 | 0.911 |
| SUVmean | 0.001 | 0.995 | 0.053 | 0.687 |
| HI1(max-min/mean) | 0.187 | 0.254 | −0.377 | 0.003 |
| MTV %42 | −0.323 | 0.045 | −0.122 | 0.350 |
| TLG | −0.194 | 0.237 | −0.107 | 0.410 |
| SUVpeak | −0.055 | 0.738 | 0.015 | 0.908 |
| HI2(MTV threshold index) | −0.216 | 0.187 | −0.202 | 0.119 |
| HI3 (% HI2) | −0.018 | 0.911 | −0.333 | 0.009 |
| Liver SUVmax | −0.153 | 0.351 | 0.156 | 0.231 |
| Liver SUVmean | −0.054 | 0.746 | 0.152 | 0.242 |
| Spleen SUVmax | 0.036 | 0.830 | 0.173 | 0.183 |
| Spleen SUVmean | 0.101 | 0.542 | 0.202 | 0.119 |
| BM SUVmax | −0.136 | 0.408 | 0.116 | 0.374 |
| BM SUVmean | −0.024 | 0.883 | 0.143 | 0.271 |
| SLR SUVmax | 0.027 | 0.872 | −0.008 | 0.952 |
| SLR SUVmean | 0.135 | 0.412 | −0.012 | 0.929 |
| BLR SUVmax | −0.117 | 0.478 | −0.010 | 0.937 |
| BLR SUVmean | −0.003 | 0.983 | 0.020 | 0.881 |
Correlation of TILs with all parameters in HER 2 and TNBC groups.
Spearman Correlation Analysis.
TILs, tumor-infiltrating lymphocytes; T, Tumor; N: lymph node; M, Metastasis; SUVmax, maximum standardized uptake value; SUVmin, minimum standardized uptake value; SUVmean, mean standardized uptake value; HI, Heterogeneity Index; MTV, metabolic tumor volume; TLG, total lesion glycolysis; SUVpeak, peak standardized uptake value; SLR, spleen-to-liver ratio; BLR, bone marrow-to-liver ratio.
The tumor size, HI1, HI2, HI3, and BLR SUVmean ratio were found to be statistically significantly higher in patients with shorter survival (p = 0.040 p = 0.048 p = 0.007 p = 0.013 p = 0.012) (Table 5).
TABLE 5
| p | HR | %95 CI | |||
|---|---|---|---|---|---|
| Enter Method | TNBC1HER20 | 0.382 | 2.898 | 0.267 | 31.424 |
| Stage (Ref:2) | 0.000 | ||||
| Stage 3 | 0.239 | 3.172 | 0.465 | 21.647 | |
| Stage 4 | <0.001 | 69.19 | 11.33 | 422.3 | |
| Axillary lymph nodes positive | 0.833 | 0.848 | 0.182 | 3.945 | |
| Diameter | 0.198 | 1.036 | 0.982 | 1.093 | |
| SUVmin | 0.647 | 0.815 | 0.340 | 1.955 | |
| SUVmean | 0.588 | 0.863 | 0.507 | 1.470 | |
| HI1 | 0.232 | 0.057 | 0.001 | 6.215 | |
| MTV42 | 0.149 | 0.951 | 0.887 | 1.018 | |
| TLG | 0.766 | 1.001 | 0.995 | 1.007 | |
| HI2 MTV threshold index | 0.391 | 1.175 | 0.813 | 1.697 | |
| HI3HI2 | 0.046 | 3.366 | 1.023 | 11.076 | |
| Liver SUVmean | 0.366 | 1.406 | 0.671 | 2.943 | |
| BLR SUV max | 0.839 | 1.450 | 0.041 | 51.816 | |
| BLR SUV mean | 0.456 | 4.646 | 0.082 | 264.150 | |
| TILs | 0.688 | 1.006 | 0.977 | 1.036 | |
| Backward Method | Stage (Ref:2) | 0.000 | |||
| Stage 3 | 0.223 | 2.434 | 0.581 | 10.190 | |
| Stage 4 | <0.001 | 32.88 | 9.086 | 118.99 | |
| SUVmin | 0.006 | 0.764 | 0.631 | 0.925 | |
| HI3HI2 | 0.011 | 2.448 | 1.230 | 4.870 | |
| BLR SUVmean | 0.043 | 4.593 | 1.052 | 20.055 | |
Univariate analysis to examine the risk effect of TILs on exitus.
HER2, Human epidermal growth factor receptor-2; TNBC, triple-negative breast cancer; SUVmax, maximum standardized uptake value; SUVmin, minimum standardized uptake value; SUVmean, mean standardized uptake value; HI, Heterogeneity Index; MTV, metabolic tumor volume; TLG, total lesion glycolysis; BLR, bone marrow-to-liver ratio; TILs, tumor-infiltrating lymphocytes.
In the model created using variables identified with p < 0.250 in univariate analyses, the backward method revealed that Ki67, MTV %42, and multicentricity were statistically significant factors (p = 0.017, p = 0.030, p = 0.011) (Table 6).
TABLE 6
| p | Beta | B | %95 CI | |||
|---|---|---|---|---|---|---|
| Model | Fixed | 18.883 | −4.871 | 42.637 | ||
| Ki67 | 0.186 | 0.145 | 0.129 | −0.063 | 0.320 | |
| Stage | 0.793 | −0.028 | −0.290 | −2.482 | 1.903 | |
| MTV %42 | 0.310 | −0.168 | −0.130 | −0.384 | 0.123 | |
| HI2(MTV threshold index) | 0.755 | −0.051 | −0.353 | −2.598 | 1.892 | |
| HI3 (% HI2) | 0.145 | −0.155 | −4.012 | −9.437 | 1.412 | |
| Spleen SUVmax | 0.162 | −0.445 | −14.051 | −33.866 | 5.765 | |
| Spleen SUVmean | 0.116 | 0.488 | 18.578 | −4.681 | 41.837 | |
| BM SUVmean | 0.427 | 0.114 | 2.729 | −4.072 | 9.531 | |
| TNBC1 HER2 0 | 0.441 | 0.094 | 3.887 | −6.084 | 13.859 | |
| Multicentricity | 0.052 | −0.202 | −8.881 | −17.831 | 0.069 | |
| R Square | 0.213 | |||||
| Backward Method | Fixed | 12.685 | 2.619 | 22.751 | ||
| Ki67 | 0.017 | 0.239 | 0.212 | 0.039 | 0.385 | |
| MTV %42 | 0.030 | −0.218 | −0.169 | −0.322 | −0.017 | |
| Multicentricity | 0.011 | −0.248 | −10.902 | −19.266 | −2.537 | |
| R Square | 0.138 | |||||
| Dependent Variable: TILs | ||||||
Multivariate linear regression analysis.
MTV: metabolic tumor volume; HI: heterogeneity index; SUVmax: maximum standardized uptake value; SUVmean: mean standardized uptake value; BM SUV, mean: Bone marrow mean standardized uptake value; TNBC: triple-negative breast cancer; HER2: Human epidermal growth factor receptor-2.
To examine the risk effect of the Tumor-Infiltrating Lymphocyte index on mortality, a model was created using variables identified with p < 0.250 in univariate analyses and analyzed using the backward method. In this analysis, Stage 4 compared to Stage 2, HI3, HI2, BLR SUV mean increase was found to be risk factors, and SUVmin increase was found to be protective statistically significant factors (p < 0.01 p = 0.011 p = 0.043 p = 0.006).
The ROC curve analysis performed to determine the TIL cut-off value for Stage 4 determined as <5 with a sensitivity of 33.3%, specificity of 82.9%, positive predictive value of 38.1%, negative predictive value of 79.7%, and overall accuracy rate of 71% (Figure 3). The characteristics of patients with TIL levels of 0–10, 10–40, and >40 are shown in Table 7.
FIGURE 3
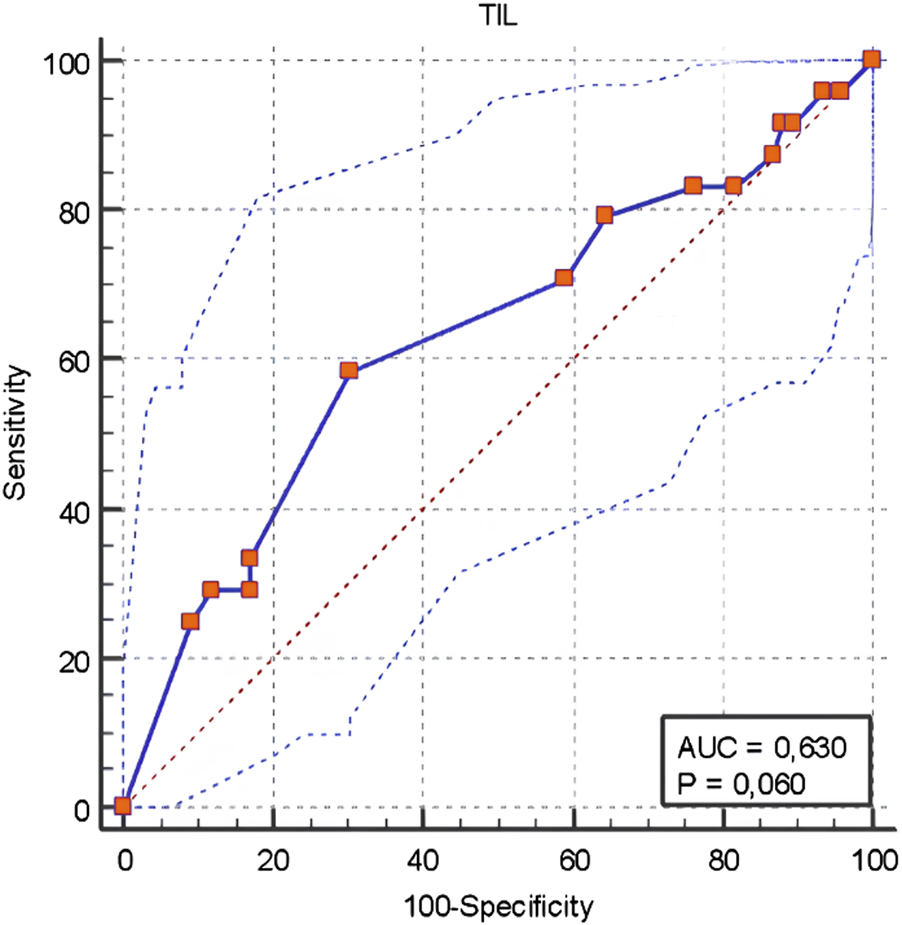
ROC Curve Analysis for the TIL cut-off value determining Stage 4.
TABLE 7
| TILs | pa | |||||||
|---|---|---|---|---|---|---|---|---|
| 0–10 | 10–40 | >40 | ||||||
| n | % | n | % | n | % | |||
| TNBC/HER2 | HER2 | 27 | 43.5 | 11 | 40.7 | 1 | 9.1 | 0.095 |
| TNBC | 35 | 56.5 | 16 | 59.3 | 10 | 90.9 | ||
| Nuclear grade | 2 | 3 | 4.8 | 2 | 7.4 | 0 | 0.0 | 0.804 |
| 3 | 59 | 95.2 | 25 | 92.6 | 11 | 100.0 | ||
| T | 1 | 1 | 1.6 | 1 | 3.7 | 0 | 0.0 | 0.713 |
| 2 | 40 | 64.5 | 18 | 66.7 | 10 | 90.9 | ||
| 3 | 13 | 21.0 | 6 | 22.2 | 1 | 9.1 | ||
| 4 | 8 | 12.9 | 2 | 7.4 | 0 | 0.0 | ||
| N | 0 | 17 | 27.4 | 7 | 25.9 | 2 | 18.2 | 0.166 |
| 1 | 16 | 25.8 | 6 | 22.2 | 4 | 36.4 | ||
| 2 | 7 | 11.3 | 8 | 29.6 | 4 | 36.4 | ||
| 3 | 22 | 35.5 | 6 | 22.2 | 1 | 9.1 | ||
| M | 0 | 45 | 72.6 | 23 | 85.2 | 9 | 81.8 | 0.397 |
| 1 | 17 | 27.4 | 4 | 14.8 | 3 | 18.2 | ||
| Stage | 2A | 15 | 24.2 | 8 | 29.6 | 2 | 18.2 | 0.434 |
| 2B | 10 | 16.1 | 2 | 7.4 | 3 | 27.3 | ||
| 3A | 5 | 8.1 | 7 | 25.9 | 3 | 27.3 | ||
| 3B | 4 | 6.5 | 0 | 0.0 | 0 | 0.0 | ||
| 3C | 11 | 17.7 | 5 | 18.5 | 1 | 9.1 | ||
| 4 | 17 | 27.4 | 5 | 18.5 | 2 | 18.2 | ||
| Multicentricity | negative | 40 | 64.5 | 19 | 70.4 | 11 | 100 | 0.061 |
| Positive | 22 | 35.5 | 8 | 29.6 | 0 | 0.0 | ||
| Axillary Lymph node | negative | 17 | 33.9 | 7 | 33.3 | 2 | 18.2 | 0.581 |
| Positive | 45 | 66.1 | 20 | 66.7 | 9 | 81.8 | ||
Disease characteristics in TILs groups.
Chi Square Test.
TILs, tumor-infiltrating lymphocytes; TNBC, triple-negative breast cancer; HER2, Human epidermal growth factor receptor-2; T, Tumor; N, lymph node; M, metastasis.
Patients with stages T3-4 or M1 had a shorter overall survival rate (p = 0.033, p < 0.001, p < 0.001). The overall survival rates of the TIL groups did not differ substantially (p = 0.283) (Figure 4). In the TNBC and HER2 groups, there was no statistically significant difference in overall survival between the TIL groups (p = 0.322, p = 0.646). The survival rates of metastatic and non-metastatic patients in the TIL groups were not substantially different (p = 0.333, p = 0.786). Patients having a TIL cut-off value of <5 had significantly worse survival rates compared to those with a TIL value of ≥5 (p = 0.031) (Figure 5).
FIGURE 4
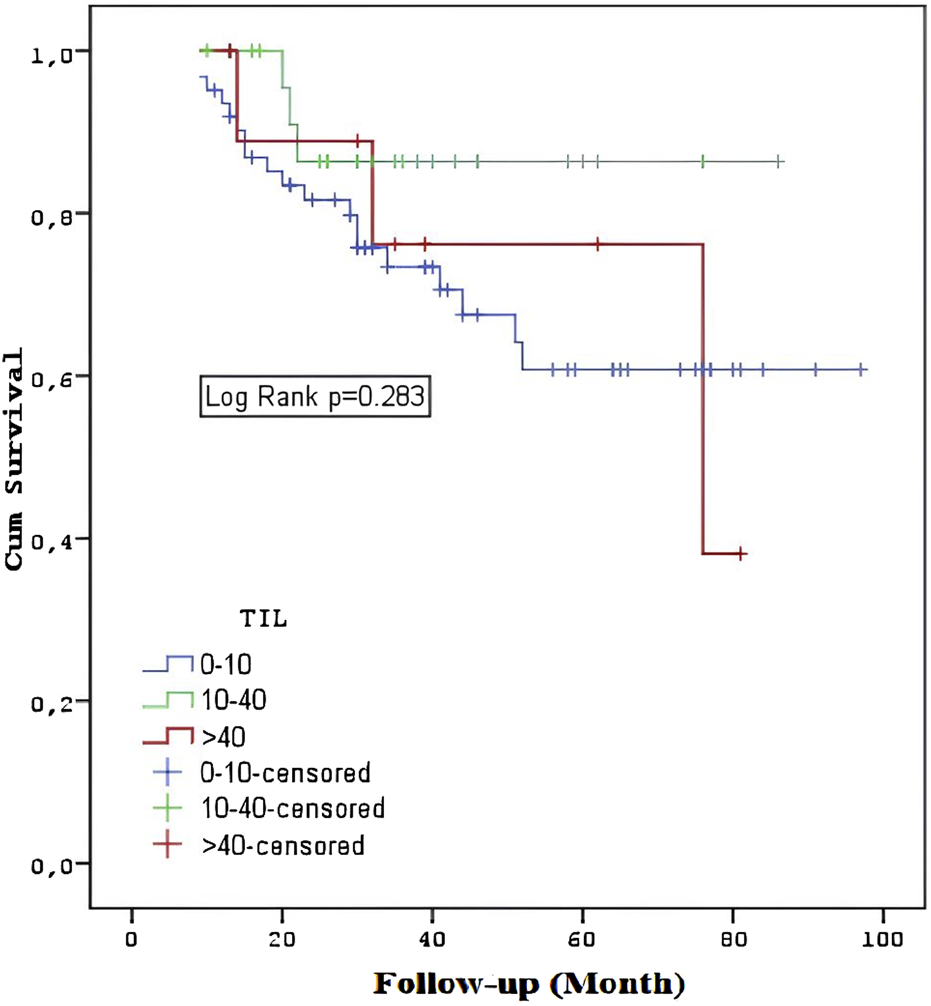
Overall survival among the TIL groups.
FIGURE 5
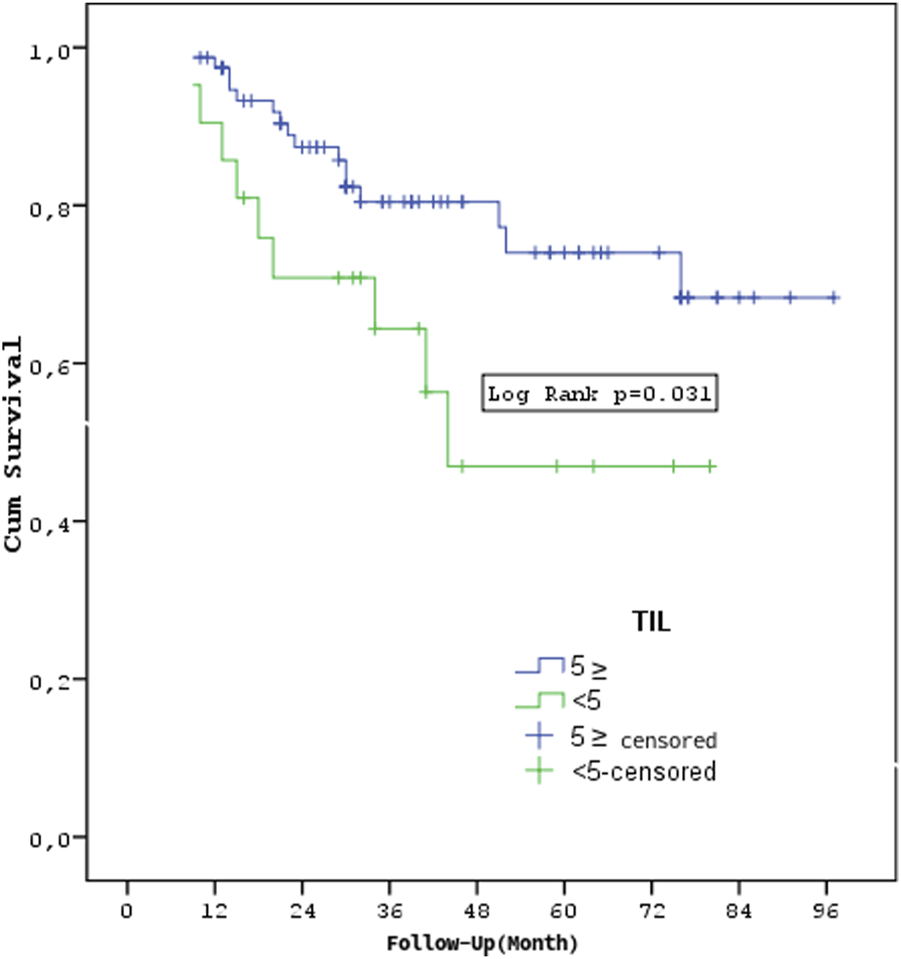
Survival according to TIL cut-off value.
Discussion
This study examined the relationship between tumor-infiltrating lymphocyte levels, FDG metabolic parameters, and tumor heterogeneity in non-luminal breast cancers and their relationship with overall survival. We found a significant relationship between TIL levels and FDG metabolic parameters in non-luminal breast cancers, especially in advanced-stage disease. Our data showed that TIL levels had moderate positive correlations with liver SUVmean, spleen SUVmax and SUVmean, and bone marrow SUVmax and SUVmean in Stage 4 patients. (p = 0.004, p = 0.007, p = 0.009). We also found that BLR SUV mean values were significantly higher in patients with short survival.
The role of TILs and 18F-FDG PET/CT metabolic characteristics in this advanced stage can shed light on the disease’s biology and potential therapeutic approaches. Higher TIL levels are linked to increased metabolic activity, implying that metabolic parameters could be useful prognostic markers [10, 11, 14, 22]. Further research is required on this topic.
Contrary to previous research by Murakami et al., which reported a positive correlation between 18F-FDG uptake and increased TIL levels indicated by higher SUVmax values, our study did not find a significant correlation between TIL levels and SUV values in non-luminal breast cancers [8]. Kitajima et al. also reported a positive correlation between TIL levels and SUVmax of FDG-PET in breast cancer patients, suggesting that SUVmax plays an important role in prognosis in less aggressive and immune-ineffective microenvironments such as SUVmax-low, TIL-low, or luminal subtype breast cancer [10]. Sasada et al. indicated that while both whole-body PET/CT and dedicated breast PET/CT could measure SUVmax to assess TIL levels, a high lymphocytic infiltrate was associated with higher SUVmax values only in dedicated breast PET, making it potentially more effective in evaluating the immune microenvironment, particularly in early-stage disease [23]. Similar findings were reported by Park et al., who noted a correlation between higher SUVmax values and higher TIL levels, and Hirakata et al., who also found a strong correlation between high TIL levels and high SUVmax [12, 24]. However, Kajary et al. reported no significant differences in kinetic parameters when comparing breast tumors with varying levels of stromal TIL, suggesting that stromal TIL does not significantly impact measured FDG kinetics [25].
Furthermore, new research has revealed spleen and bone marrow FDG uptake as critical biomarkers for determining the systemic immune response to malignancy. Increased FDG uptake in these organs may indicate an activated or stressed immune system, which is frequently associated with a poor prognosis due to systemic inflammation or immunological dysregulation, especially in advanced disease stages [11, 13–15]. Seban et al. have found that spleen glucose metabolism may be a stronger predictor of prognosis than bone marrow metabolism, with total metabolic tumor volume and spleen-to-liver glucose uptake ratio helping as independent prognostic indicators for predicting 5-year recurrence in breast cancer [11].
In our study, although no statistical correlation was observed between TIL levels and SUV values in non-luminal breast cancer, heterogeneity indices (HI2 and HI3) emerged as significant risk factors for mortality. This finding is consistent with previous studies highlighting the predictive value of FDG intratumoral heterogeneity in determining disease characteristics and survival outcomes across different cancer types [11, 16, 17, 26, 27]. Our results further revealed that TIL levels had a weak negative correlation with HI1 and HI3 in TNBC patients, while no correlation was observed in the HER2-positive group. Moreover, in stage 4 non-luminal breast cancer, TIL levels showed a moderately negative correlation with HI2 and HI3, indicating that higher TIL levels might correlate with less aggressive histological features even in advanced disease stages.
Interestingly, in our study, HI1, HI2, and HI3 values were found to be significantly higher in patients with short survival, while no statistically significant difference was found in survival rates with TIL groups (p = 0.283). In addition, no statistically significant difference was found in survival rates of TIL groups in TNBC and HER2 groups (p = 0.322 p = 0.646). This finding suggests a more complex relationship between TIL levels and patient outcomes. TIL may not be the sole determinant of survival, but it still plays a role in modulating tumor behavior and response to treatment [5, 10, 14, 15].
Previous research has shown that TIL, particularly when measured before chemotherapy, is related to a better prognosis and can predict the therapeutic impact of neoadjuvant chemotherapy in breast cancer [5, 8, 9]. However, in our investigation, patients with TIL levels less than 5 had significantly lower survival rates than those with TIL levels 5 or higher (p = 0.031), which is consistent with previous research suggesting that low TIL levels are associated with poorer outcomes across various breast cancer subtypes [6, 28].
This study’s limitations include its retrospective design, a small sample size from a single center, the use of biopsy samples for TIL assessment, classification by a single pathologist, and the lack of evaluation of treatments received or progression-free survival.
In conclusion, our study demonstrates a significant relationship between TIL, FDG metabolic parameters, and tumor heterogeneity in non-luminal breast cancers, especially in advanced stages. We found positive correlations between TIL levels and metabolic activity in critical organs like the liver, spleen, and bone marrow, which may serve as valuable prognostic indicators. Additionally, heterogeneity indices emerged as risk factors for mortality. These findings suggest that TIL levels and metabolic parameters offer deeper insights into tumor behavior and prognosis. Further research involving larger cohorts and diverse breast cancer subtypes is necessary to validate these findings and better understand the interplay between tumor metabolism and survival.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the study was authorized by the local ethics committee of Prof. Dr. Cemil Tascioglu City Hospital in Istanbul, Turkey (2023/254), and informed consent was received from all participating patients. All procedures in human-participant studies complied with the institutional research committee’s ethical standards and the Helsinki Declaration of 1964. The studies were conducted in accordance with the local legislation and institutional requirements.
Author contributions
MT and HO participated in the study design, drafting of the manuscript, and data acquisition and analysis. GB, MNAT, and HO participated in data acquisition and data analysis. MNAT, MT, and GK participated in the study conception and design, data analysis, manuscript revision, and approval of the final content of the manuscript. All authors read and approved the final manuscript.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Abbreviations
NLBC, Non-luminal breast cancers; HER2, Human epidermal growth factor receptor-2; TNBC, Triple-negative breast cancer; TILs, Tumor-infiltrating lymphocytes; 18F-FDG, Fluorine-18 fluorodeoxyglucose; PET/CT, Positron emission tomography-computed tomography; T, Tumor; N, Lymph Node; M, Metastasis; SUVmax, Maximum standardized uptake value; SUVmin, Minimum standardized uptake value; SUVmean, Mean standardized uptake value; HI, Heterogeneity Index; MTV, Metabolic tumor volume; TLG, Total Lesion Glycolysis; SUVpeak, Peak standardized uptake value; SLR, Spleen-to-liver ratio; BLR, Bone marrow-to-liver ratio; IHC, Immunohistochemistry.
References
1.
Denkert C Minckwitz G Darb-Esfahani S Lederer B Heppner B Weber K et al Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol (2018) 19:40–50. 10.1016/S1470-2045(17)30904-X
2.
Goldhirsch A Wood WC Coates AS Thürlimann B Senn H-J Senn HJ et al Strategies for subtypes – dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer. Ann Oncol (2011) 22:1736–47. 10.1093/annonc/mdr304
3.
Salgado R Denkert C Demaria NS Sirtaine N Klauschen F Pruneri G et al The evaluation of tumor-infiltrating lymphocytes (TILS) in breast cancer: recommendations by an International TILS Working Group 2014. Ann Oncol (2015) 26:259–71. 10.1093/annonc/mdu450
4.
Ciarka A Piatek M Peksa R Kunc M Senkus E . Tumor-infiltrating lymphocytes (TILs) in breast cancer: prognostic and predictive significance across molecular subtypes. Biomedicines (2024) 12(4):763. 10.3390/biomedicines12040763
5.
Yu X Zhang Z Wang Z Wu P Qiu F Huang J . Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin Transl Oncol (2016) 18(5):497–506. 10.1007/s12094-015-1391-y
6.
Althobiti M Aleskandarany MA Joseph C Toss M Mongan N Diez-Rodriguez M et al Heterogeneity of tumor-infiltrating lymphocytes in breast cancer and its prognostic significance. Histopathology (2018) 73(6):887–96. 10.1111/his.13695
7.
Saber MM El Zawahry HM Hilal AM Abou-Bakr A Namour AE Sabere MM et al Prognostic value of tumor infiltrating lymphocytes in locally advanced HER2 enriched breast cancer. Asian Pac J Cancer Prev (2022) 23(2):553–60. 10.31557/APJCP.2022.23.2.553
8.
Murakami W Tozaki M Sasaki M Hida A Ohi Y Kubota K et al Correlation between 18F-FDG uptake on PET/MRI and the level of tumor-infiltrating lymphocytes (TILs) in triple-negative and HER2-positive breast cancer. Eur J Radiol (2020) 123:108773. 10.1016/j.ejrad.2019.108773
9.
de Moraes FCA Souza MEC Sano VKT Moraes RA Melo AC . Association of tumor-infiltrating lymphocytes with clinical outcomes in patients with triple-negative breast cancer receiving neoadjuvant chemotherapy: a systematic review and meta-analysis. Clin Transl Oncol (2024)). 10.1007/s12094-024-03661-8
10.
Kitajima K Higuchi T Fujimoto Y Ishikawa E Yokoyama H Komoto H et al Relationship between FDG-PET and the immune microenvironment in breast cancer. Eur J Radiol (2023) 158:110661. 10.1016/j.ejrad.2022.110661
11.
Seban RD Rouzier R Latouche A Deleval N Guinebretiere JM Buvatet I et al Total metabolic tumor volume and spleen metabolism on baseline [18F]-FDG PET/CT as independent prognostic biomarkers of recurrence in resected breast cancer. Eur J Nucl Med Mol Imaging (2021) 48(11):3560–70. 10.1007/s00259-021-05322-2
12.
Park S Min EK Bae SJ Cha C Kim D Lee J et al Relationship of the standard uptake value of (18)F-FDG-PET-CT with tumor-infiltrating lymphocytes in breast tumors measuring 1 cm. Sci Rep (2021) 11(1):12046. 10.1038/s41598-021-91404-y
13.
Lee JW Kim SY Han SW Lee JE Lee HJ Heo NH et al [18F]FDG uptake of bone marrow on PET/CT for predicting distant recurrence in breast cancer patients after surgical resection. EJNMMI Res (2020) 10(1):72. 10.1186/s13550-020-00660-y
14.
Wang Y Li Y Jiang H Zuo C Xu W . Elevated splenic 18F-fluorodeoxyglucose positron emission tomography/computed tomography activity is associated with 5-year risk of recurrence in non-metastatic invasive ductal carcinoma of the breast. Br J Radiol (2024) 97(1153):237–48. 10.1093/bjr/tqad015
15.
Bang JI Yoon HJ Kim BS . Clinical utility of FDG uptake within reticuloendothelial system on F-18 FDG PET/CT for prediction of tumor recurrence in breast cancer. PLoS One (2018) 13(12):e0208861. 10.1371/journal.pone.0208861
16.
Liu G Yin H Cheng X Wang Y Hu Y Liu T et al Intra-tumor metabolic heterogeneity of gastric cancer on 18F-FDG PETCT indicates patient survival outcomes. Clin Exp Med (2021) 21:129–38. 10.1007/s10238-020-00659-8
17.
Huang B Chan T Kwong DL-W Chan WKS Khong PL . Nasopharyngeal carcinoma: investigation of intratumoral heterogeneity with FDG PET/CT. Am J Roentgenol (2012) 199:169–74. 10.2214/AJR.11.7336
18.
Babacan GB Tamam MO Saracoglu S Acar Tayyar MN Sahin MC Kulduk G et al Can tumor heterogeneity in TNBC obtained with FDG PET/CT predict survival? Novel method: heterogeneity index 3. Eur J Nucl Med Mol Imaging (2023) 50(Suppl. 1):S478–S898. 10.1007/s00259-023-06333-x
19.
Sasada S Kimura Y Emi A Masumoto N Kadoya T Arihiro K et al Tumor-infiltrating lymphocyte score based on FDG PET/CT for predicting the effect of neoadjuvant chemotherapy in breast cancer. Anticancer Res (2020) 40(6):3395–400. 10.21873/anticanres.14323
20.
Giuliano AE Connolly JL Edge SB Mittendorf EA Rugo HS Solin LJ et al Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin (2017) 67:290–303. 10.3322/caac.21393
21.
Wolff AC Hammond MEH Schwartz JN Hagerty KL Allred DC Cote RJ et al American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol (2007) 25(1):118–45. 10.1200/JCO.2006.09.2775
22.
Frankowska K Zarobkiewicz M Dąbrowska I Bojarska-Junak A . Tumor infiltrating lymphocytes and radiological picture of the tumor. Med Oncol (2023) 40(6):176. 10.1007/s12032-023-02036-3
23.
Sasada S Shiroma N Goda N Kajitani K Emi A Masumoto N et al The relationship between ring-type dedicated breast PET and immune microenvironment in early breast cancer. Breast Cancer Res Treat (2019) 177:651–7. 10.1007/s10549-019-05339-0
24.
Hirakata T Fujii T Kurozumi S Katayama A Honda C Yanai K et al FDG uptake reflects breast cancer immunological features:the PD-L1 expression and degree of TILs in primary breast cancer. Breast Cancer Res Treat (2020) 181:331–8. 10.1007/s10549-020-05619-0
25.
Kajáry K Lengyel Z Tőkés A-M Kulka J Dank M Tőkés T . Dynamic FDG-PET/CT in the initial staging of primary breast cancer: clinicopathological correlations. Pathol Oncol Res (2020) 26:997–1006. 10.1007/s12253-019-00641-0
26.
Kim Y Kim YJ Paeng JC Cheon GJ Lee DS Chung JK et al Heterogeneity index evaluated by slope of linear regression on 18F-FDG PET/CT as a prognostic marker for predicting tumor recurrence in pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging (2017) 44:1995–2003. 10.1007/s00259-017-3755-8
27.
Li R Lin J Wang L Zheng X Tang K . The association between 18F-fluorodeoxyglucose PET intratumoral metabolic heterogeneity and pathological parameters in non-small cell lung cancer. Nucl Med Commun (2019) 40:1022–8. 10.1097/MNM.0000000000001058
28.
Park HS Heo I Kim JY Kim S Nam S Park S et al No effect of tumor-infiltrating lymphocytes (TILs) on prognosis in patients with early triple-negative breast cancer: validation of recommendations by the International TILs Working Group 2014. J Surg Oncol (2016) 114(1):17–21. 10.1002/jso.24275
Summary
Keywords
tumor-infiltrating lymphocytes, nonluminal breast cancer, tumor heterogeneity indices, FDG, metabolic parameter
Citation
Tamam M, Ozcevik H, Kulduk G, Acar Tayyar MN and Babacan GB (2025) Evaluating the correlation between pretreatment 18F-FDG PET/CT metabolic parameters and tumor-infiltrating lymphocyte levels in nonluminal breast cancer and impact on survival. Pathol. Oncol. Res. 30:1612014. doi: 10.3389/pore.2024.1612014
Received
28 October 2024
Accepted
20 December 2024
Published
08 January 2025
Volume
30 - 2024
Edited by
Anna Tokes, Semmelweis University, Hungary
Updates
Copyright
© 2025 Tamam, Ozcevik, Kulduk, Acar Tayyar and Babacan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muge Tamam, mugetamam@yahoo.com
ORCID: Muge Tamam, orcid.org/0000-0002-3793-0178; Halim Ozcevik, orcid.org/0000-0001-7201-9868; Gamze Kulduk, orcid.org/0000-0001-9071-651X; Merve Nur Acar Tayyar, orcid.org/0009-0001-4126-7778; Gunduzalp Bugrahan Babacan, orcid.org/0000-0002-0561-5739
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.