Abstract
Accurate lymph node (LN) retrieval during colorectal carcinoma resection is pivotal for precise N-staging and the determination of adjuvant therapy. Current guidelines recommend the examination of at least 12 mesocolic or mesorectal lymph nodes for accurate staging. Traditional histological processing techniques, reliant on visual inspection and palpation, are time-consuming and heavily dependent on the examiner’s expertise and availability. Various methods have been documented to enhance LN retrieval from colorectal specimens, including intra-arterial ex vivo methylene blue injection. Recent studies have explored the utility of indocyanine green (ICG) fluorescence imaging for visualizing pericolic lymph nodes and identifying sentinel lymph nodes in colorectal malignancies. This study included 10 patients who underwent colon resection for malignant tumors. During surgery, intravenous ICG dye and an endoscopic camera were employed to assess intestinal perfusion. Post-resection, ex vivo intra-arterial administration of ICG dye was performed on the specimens, followed by routine histological processing and an ICG-assisted lymph node dissection. The objective was to evaluate whether ICG imaging could identify additional lymph nodes compared to routine manual dissection and to assess the clinical relevance of these findings. For each patient, a minimum of 12 lymph nodes (median = 25.5, interquartile range = 12.25, maximum = 33) were examined. ICG imaging facilitated the detection of a median of three additional lymph nodes not identified during routine processing. Metastatic lymph nodes were found in four patients however no additional metastatic nodes were detected with ICG assistance. Our findings suggest that ex vivo intra-arterial administration of indocyanine green dye can augment lymph node dissection, particularly in cases where the number of lymph nodes retrieved is below the recommended threshold of 12.
Introduction
Following the resection for colorectal carcinoma, the number of retrieved regional lymph nodes is important for determining the stage and adjuvant therapy. For accurate N-staging, as many as possible, but at least 12 mesocolic or mesorectal lymph nodes need to be histologically examined [1].
In the pathological processing of colorectal specimens, the traditional dissection “performed by visual inspection and palpation” is a time-consuming method. In addition, the effectiveness of lymph node harvesting significantly depends on the experience of the examiner and the time available. The technical difficulty is well illustrated by the fact that 80% of mesenteric lymph nodes are less than 3 mm in diameter, and 50% of metastatic lymph nodes are less than 5 mm in diameter [2].
Additionally, in rectal cancer cases, post-neoadjuvant total mesorectal excision (TME) dissection results in a typically low number of extracted lymph nodes, making even the threshold of 12 hard to achieve [3].
There are many methods known in the literature to facilitate the extraction of lymph nodes from a colorectal specimen, including intra-arterial ex vivo Methylene Blue specimen loading [4], fat-clearance techniques [5], and even the employment of extra pathology assistant staff [6].
In vivo, intraoperative fluorescence imaging of subserosal-injected indocyanine green (ICG) has recently been proven to be a promising method for imaging lymphatic vessels and lymph nodes [7]. Several studies have investigated peritumoral submucosal ICG injection by endoscopy to identify sentinel lymph nodes in colorectal cancer; however, the results are still controversial [8].
ICG, on the other hand, has been introduced as an effective tool to qualitatively and quantitatively verify adequate blood supply to the resection lines during colorectal resection [9]. Under near-infrared excitation (with the help of a laser light source and image processing suitable for fluorescence detection), intravenous injection of ICG solution helps to differentiate between well- and under-perfused tissues.
ICG has a relatively short half-life in the blood (approximately 2–4 min) and is quickly distributed to various tissues through systemic circulation. Combining the two phenomena that intra-arterial dyes accumulate in mesenteric lymph nodes (as in methylene blue studies) [10], and that ICG can be used for lymph node detection, we postulated that lymph node harvesting in histologic specimen processing may be enhanced by the ICG fluorescence technique. The aim of this study was to evaluate the feasibility and efficacy of the intraarterial ICG technique to facilitate lymph node dissection in colorectal resection.
Materials and methods
In our pilot study, we enrolled ten consecutive patients with colorectal cancer who underwent colorectal resection. We used the Medtronic Elevision™ IR platform imaging system with Verdye™ indocyanine dye as a fluorophore, to perform ICG fluorescence imaging (ICG-FI). The dye in powder form was dissolved in sterile distilled water for injection according to the manufacturer’s instructions, and a bolus intravenous injection of 0.05 mg/kg ICG dye was administered during surgery before colorectal resection as a routine perfusion check. After specimen extraction, arterial cannulation and a second shot of 0.05 mg/kg dose of direct intraarterial ICG were performed ex vivo under fluorescence imaging (Figure 1).
FIGURE 1
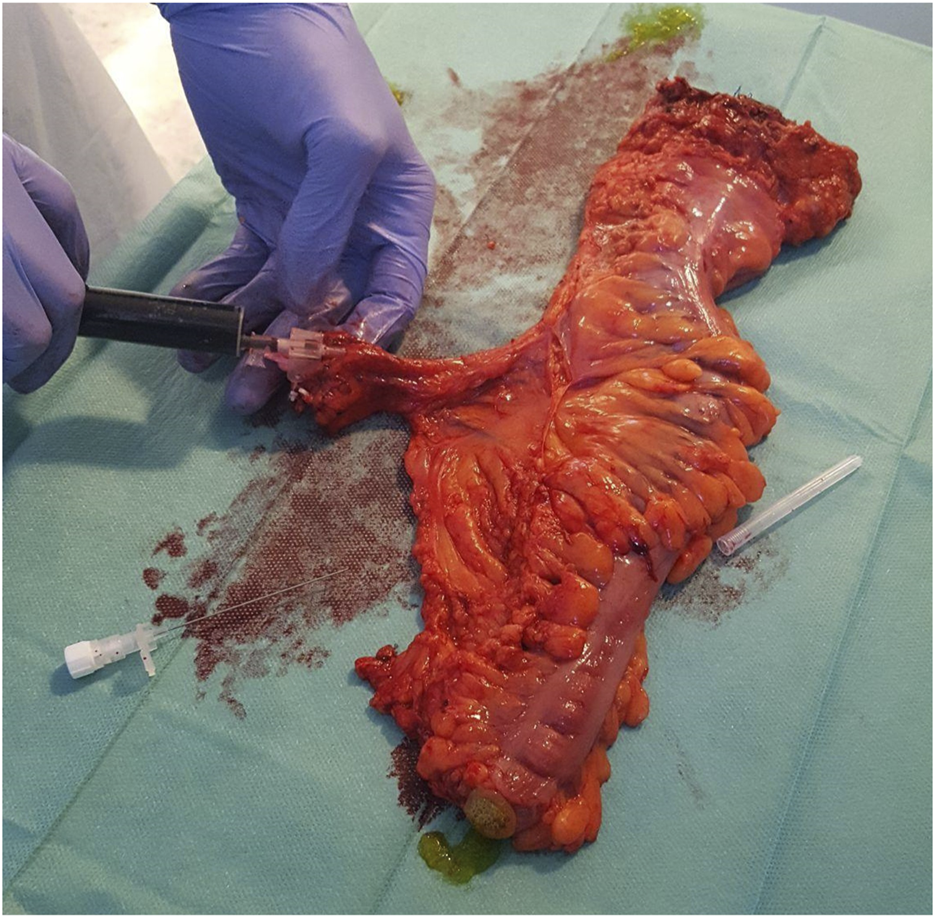
Cannulation of the supplying artery and ICG perfusion of the extracted specimen Ensure that all the figures, tables, and captions are correct, and that all figures are of the highest quality/resolution. You may upload improved figures to the Production Forum. If so, please describe in visual terms the exact changes(s) made to help us confirm that the updated version has been used in the finalized proof. Please note that figures and tables must be cited sequentially, per the author guidelines.
The specimen was then routinely processed after a 24-hour incubation in a 4% buffered formaldehyde solution and sent for traditional pathology grossing. After conventional lymph node dissection carried out by a pathologist under naked eye control and traditional palpation technique, each specimen was re-examined using ICG-FI. This was possible as formaldehyde does not compromise fluorescence excitability [11]. The additionally identified lymph nodes were sent for further pathological examination (Figure 2). The lymph node numbers of the primary (conventional) harvesting and the ICG-fluorescence-enhanced extra lymph node numbers were recorded. Lymph node sizes were measured as well during the pathological routine workup using a conventional light microscope.
FIGURE 2
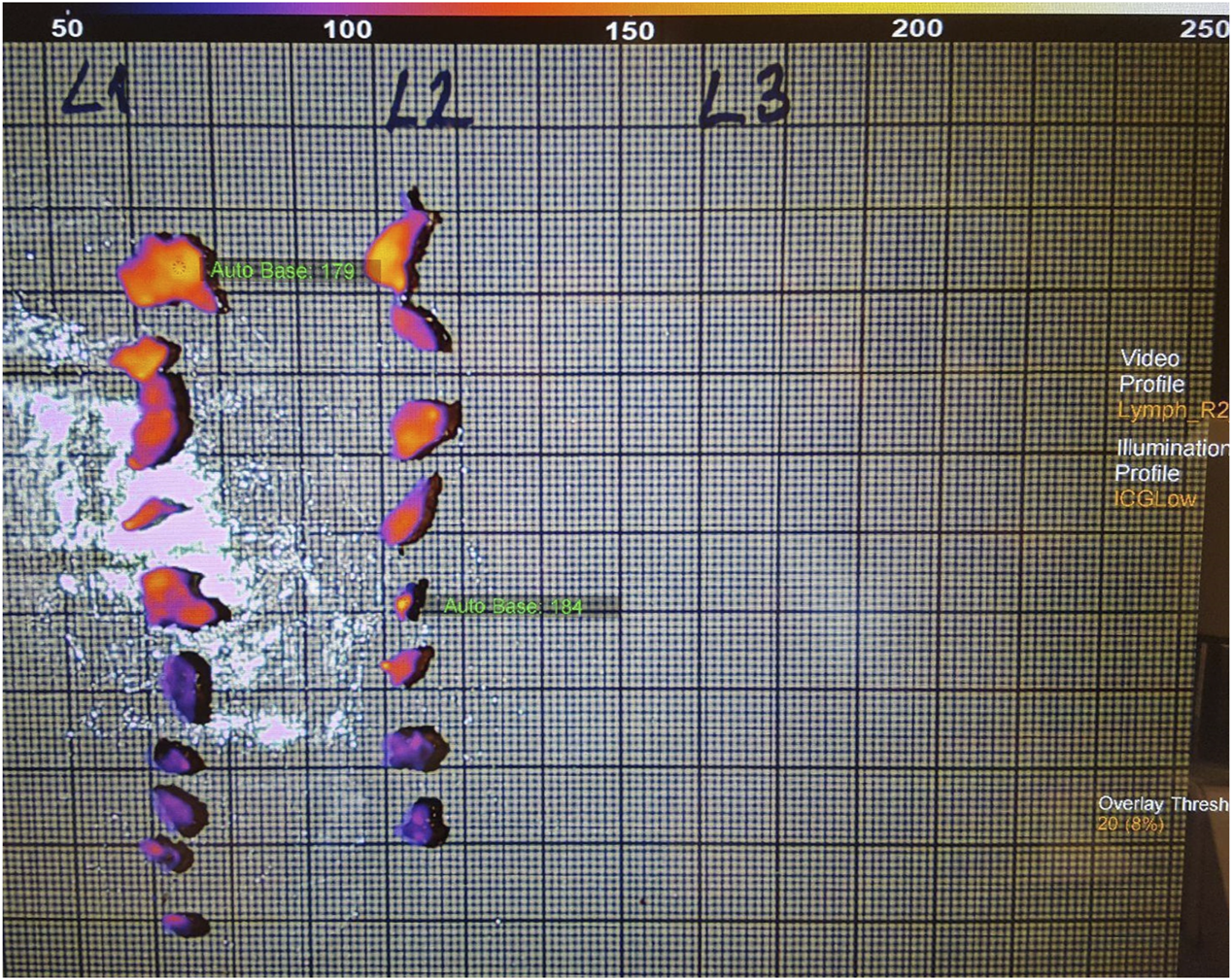
Fluorescence imaging of the extra harvested lymph nodes.
A summary of the study design is shown in Figure 3.
FIGURE 3
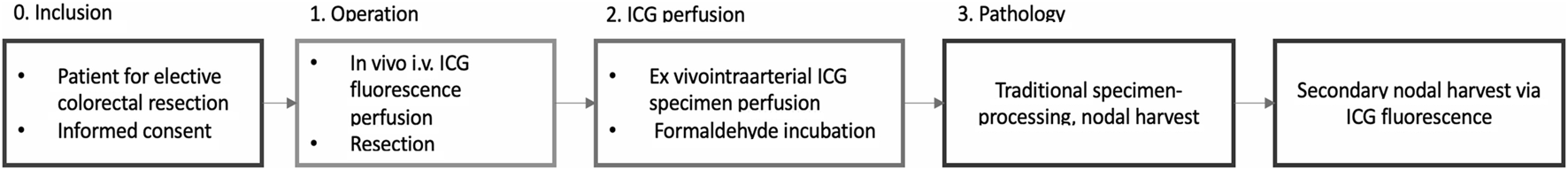
Study design.
In order to exclude or highlight the Hawthorne effect [12] we compared lymph node yields to a historical cohort of 30 consecutive patients with colorectal cancer with similar patient and clinical characteristics (propensity-matched cases).
Statistical work-up
Demographic data, cancer characteristics, lymph node numbers and lymph node sizes were analyzed. The normality of the distributions of continuous variables was tested with the Shapiro-Wilk test. Comparisons were made using the Student’s paired sample T-test for normally distributed variables and the Mann-Whitney U-test for non-Gaussian distributed ones. Differences with p < 0.05 were considered significant. The propensity score was calculated by binal logarithmic regression, covariates were T and N stages, neoadjuvant therapy and type of resection. After propensity score matching we carried out the Mann-Whitney U test using mean ranks to determine whether there was a significant increase in lymph node yield with ICG assistance in addition to traditional harvesting. Statistical analysis was performed using SPSS (IBM© SPSS© statistics 27) software.
Results
Patient characteristics and tumor characteristics are shown in Tables 1, 2.
TABLE 1
| Mean age | 73 |
| Sex ratio | 1:1 |
| Right hemicolectomy | 3 |
| Transverse colon resection | 1 |
| Rectal resection | 6 |
Patient characteristics.
TABLE 2
| Patient # | T stage | N stage | Neoadjuvant therapy |
|---|---|---|---|
| 1 | 3 | 0 | Yes |
| 2 | 4a | 1b | No |
| 3 | 3 | 1b | No |
| 4 | 1 | 0 | Yes |
| 5 | 2 | 0 | Yes |
| 6 | 3 | 2a | No |
| 7 | 2 | 0 | No |
| 8 | 2 | 0 | Yes |
| 9 | 4a | 1a | No |
| 10 | 4b | 0 | No |
Tumor characteristics.
Number of harvested lymph nodes
A median of 21.5 lymph nodes was identified in the experimental population by the initial conventional pathological workup which was supplemented by ICG fluorescence imaging to a median of 25.5 lymph nodes (Figure 6).
ICG fluorescence imaging identified an additional 34 lymph nodes in 10 cases that were not detected by traditional pathological examination (Figure 4).
FIGURE 4
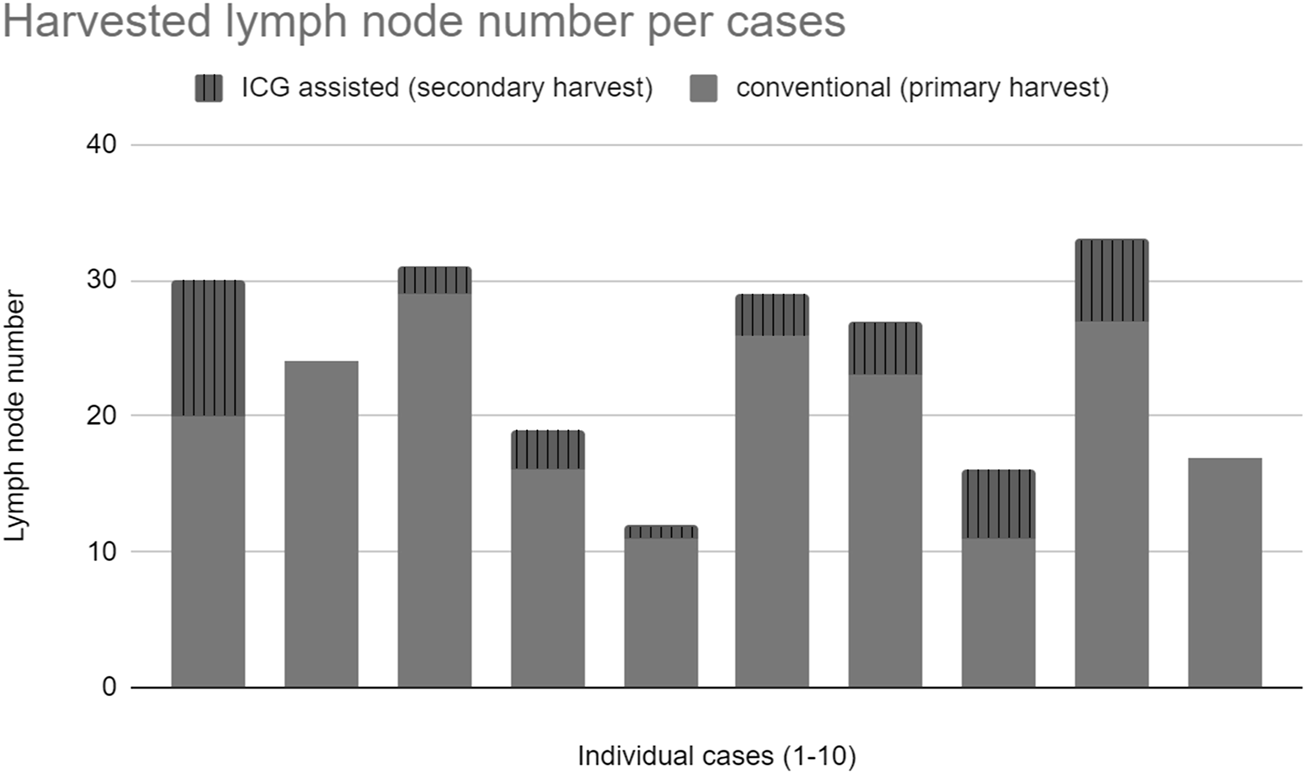
Total harvested lymph nodes.
Metastatic lymph nodes
A total of 11 metastatic lymph nodes were identified in 4 out of 10 specimens. No metastatic lymph node was identified by ICG.
Size of harvested lymph nodes
Of all metastatic lymph nodes, 50% were smaller than 5 mm in diameter. The median size of lymph nodes harvested by the traditional pathological examination was 3 mm and 1.8 mm for lymph nodes identified with ICG (Figure 5).
FIGURE 5
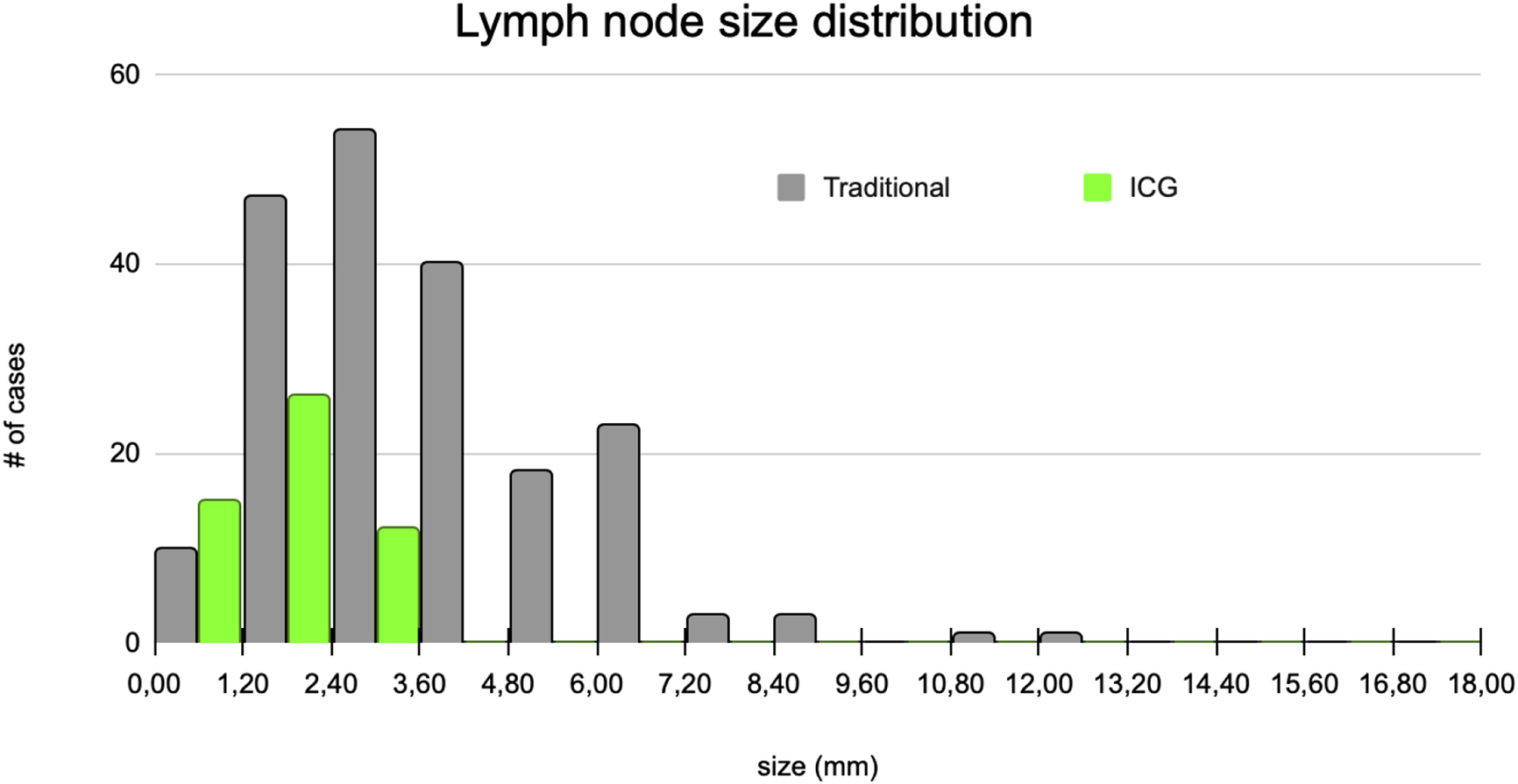
Lymph node size distributions.
The mean size of lymph nodes extracted by ICG assistance following traditional lymph node harvesting was half that of lymph nodes identified with conventional dissection (3.70 vs. 1.76 mm, SD 2.05 vs. 0.73 mm). We found that the smaller lymph nodes, which are more difficult to detect with traditional dissection methods, were more likely to be identified with ICG-FI.
Historical cohort
After propensity score matching, the median lymph node yield was significantly higher in the study group even without ICG assistance than in the control group of a historical cohort (Figure 6) (median of 21.5 vs. 13, p = 0.052, U = 24, r = 0.6). In total, 30% of the cases in the historical cohort had suboptimal nodal staging (less than 12 lymph nodes assessed), while only 2 cases (20%) had inaccurate nodal staging in the traditional work-up during the study period, both of which were improved above 12 nodes with ICG supplementation.
FIGURE 6
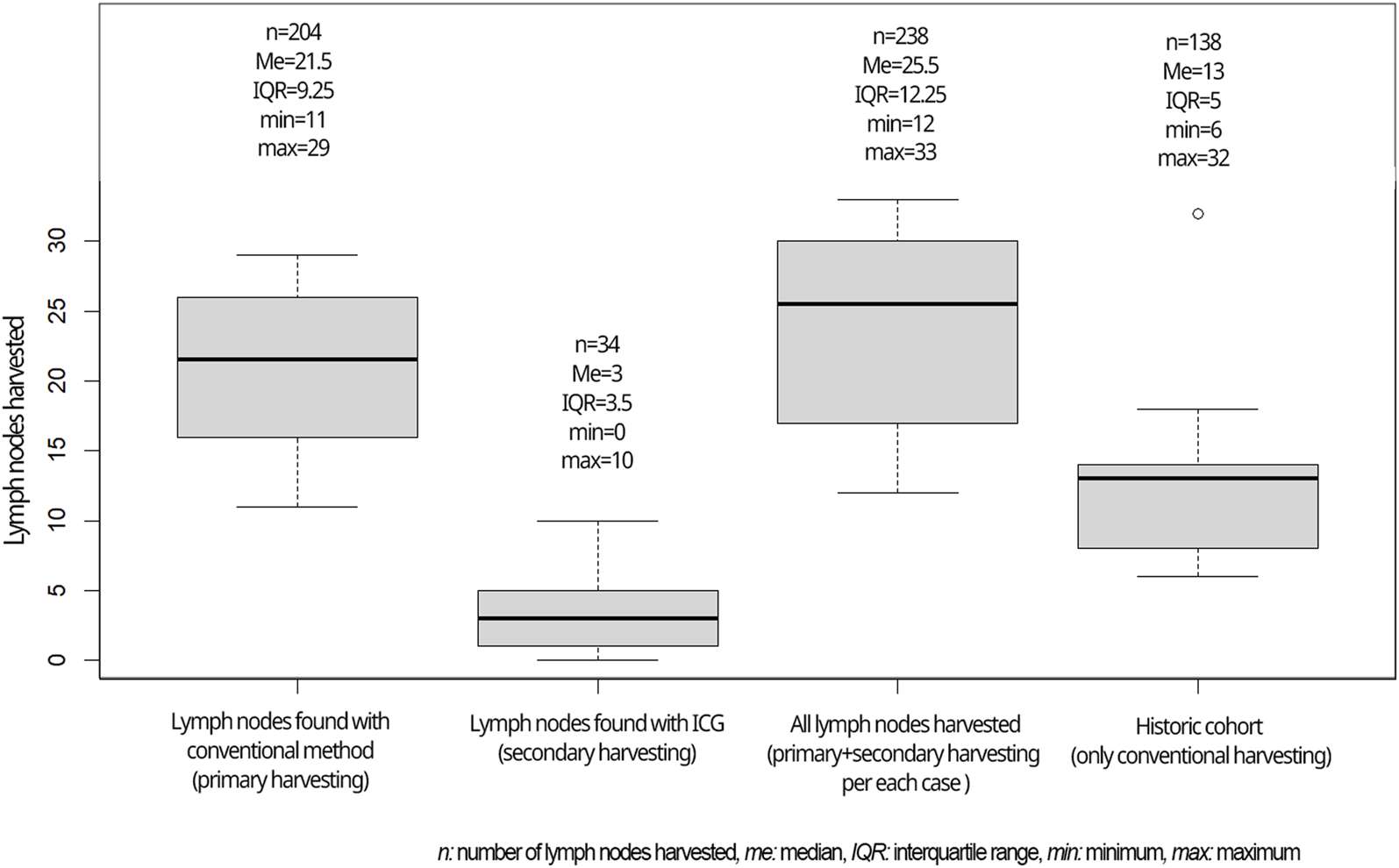
Lymph nodes harvested in the current study compared to the historical cohort.
In our current study, the accuracy was 80%, in the propensity-matched historical cohort it was 70%.
Adverse events
Because ICG-assisted lymph node dissection was performed ex vivo, no adverse events were recorded regarding the assessments. Given that a single vial of ICG is sufficient for intraoperative perfusion assessment and ex vivo lymph node identification, the technique has no additional cost.
Discussion
Indocyanine green as a fluorescent dye is commonly used in medical imaging and diagnostic procedures. It absorbs light in the near-infrared range (wavelengths of 700–900 nm), which is beyond the visible spectrum. Near-infrared light is less scattered and absorbed by tissues compared to visible light, resulting in deeper tissue penetration. The present study aimed to evaluate the feasibility and performance of ex vivo intraarterial ICG perfusion-based fluorescence lymph node identification in colorectal cancer.
The number of examined lymph nodes is highly prognostic [13] in addition to being a strong predictive factor affecting the postoperative multidisciplinary team decision regarding oncotherapy [14]. In cases of inaccurate N0 staging (examined nodes less than 12), patients may fail to receive the beneficial adjuvant therapy (which is routine in Hungarian oncological practice) or may be the target of unnecessary overtreatment (in some Western-European countries) [15].
In our pilot study of 10 consecutive cases of colorectal resection, ICG-FI significantly improved the number of retrieved lymph nodes, however the number of positive (metastatic) lymph nodes was not affected. Considering that the minimum lymph node yield for accurate staging according to the AJCC Cancer Staging System is at least 12, our data show that even without the help of ICG, this requirement is fulfilled in the majority of the current study. This makes the beneficial effect of ICG-FI even more impressive.
Our results show a significantly higher lymph node yield when compared to our retrospectively analyzed past results of conventional (manual) tissue harvesting. Therefore we have proved that the high success rate of lymph node retrieval within the study is at least partly due to a Hawthorne effect.
Consequently, the use of the ICG FI technique may be beneficial due to the higher number of identified lymph nodes, which in turn will result in more precise staging where accurate lymph node staging is more difficult to achieve on a regular basis.
Our results support the finding of other researchers that a significant proportion (up to 50%) of metastatic lymph nodes are smaller than or equal to 5 mm in diameter [16]. Therefore, the easy-to-perform ICG-FI is well expected to increase the positive lymph node yield.
Accuracy in pathological staging is defined as at least 12 non-metastatic lymph nodes found or any number of lymph nodes but with N1-2 stage [6, 17]. This is the primary requirement for the appropriate decision on the need for adjuvant chemotherapy, therefore it can also have a long-term oncological impact [18–20].
Although ICG-FI requires special equipment (laser, camera, dye), the same setup can be used for a variety of tasks. There is a good possibility that it will be used routinely both in the operating room and for pathological examinations in the future.
Conclusion
ICG fluorescence can improve the accuracy of lymph node staging. It identified additional lymph nodes that were not detected by traditional pathological examination, indicating that this technique has the potential to help optimize treatment planning and improve patient outcomes.
Centers with a lower number of routinely retrieved lymph nodes after colorectal resection, in addition to cases following neoadjuvant chemo-irradiation may benefit most from this technique.
Larger studies are needed to validate these findings and to determine the optimal protocol for using ICG fluorescence to improve histologic specimen processing after colorectal surgery.
Comparison of ICG-FI with other techniques for the same purpose (methylene blue intra-arterial injection, fat clearance techniques, use of pathology assistants, etc.) will be a task for the future.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Egészségügyi Tudományos Tanács Tudományos és Kutatásetikai Bizottság. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LL, VB, II, and AB conducted the experiments, LL and BB wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Kim J Huynh R Abraham I Kim E Kumar RR . Number of lymph nodes examined and its impact on colorectal cancer staging. The Am surgeon (2006) 72(10):902–5. 10.1177/000313480607201013
2.
Markl B Roessle J Arnholdt HM Schaller T Krammer I Cacchi C et al The clinical significance of lymph node size in colon cancer. Mod Pathol (2012) 25:1413–22. 10.1038/modpathol.2012.92
3.
Destri GL Di Carlo I Scilletta R Scilletta B Puleo S . Colorectal cancer and lymph nodes: the obsession with the number 12. World J Gastroenterol WJG (2014) 20(8):1951–60. 10.3748/wjg.v20.i8.1951
4.
Borowski DW Banky B Banerjee AK Agarwal AK Tabaqchali MA Garg DK et al Intra-arterial methylene blue injection into ex vivo colorectal cancer specimens improves lymph node staging accuracy: a randomized controlled trial. Colorectal Dis (2014) 16(9):681–9. PMID: 24911342. 10.1111/codi.12681
5.
Gregurek SF Wu HH . Can GEWF solution improve the retrieval of lymph nodes from colorectal cancer resections?Arch Pathol Lab Med (2009) 133(1):83–6. PMID: 19123742. 10.1043/1543-2165-133.1.83
6.
Schofield JB Mounter NA Mallett R Haboubi NY . The importance of accurate pathological assessment of lymph node involvement in colorectal cancer. Colorectal Dis (2006) 8(6):460–70. 10.1111/j.1463-1318.2006.01044.x
7.
Chand M Keller DS Joshi HM Devoto L Rodriguez-Justo M Cohen R . Feasibility of fluorescence lymph node imaging in colon cancer: FLICC. Tech Coloproctol (2018) 22(4):271–7. Epub 2018 Mar 17. PMID: 29551004. 10.1007/s10151-018-1773-6
8.
Sposito C Maspero M Belotti P Simonotti N Altomare M Ciana P et al Indocyanine green fluorescence-guided surgery for gastrointestinal tumors: a systematic review. Ann Surg Open (2022) 3(3):e190. PMID: 37601143; PMCID: PMC10431291. 10.1097/AS9.0000000000000190
9.
Watanabe J Ota M Suwa Y Suzuki S Suwa H Momiyama M et al Evaluation of the intestinal blood flow near the rectosigmoid junction using the indocyanine green fluorescence method in a colorectal cancer surgery. Int J colorectal Dis (2015) 30:329–35. 10.1007/s00384-015-2129-6
10.
Märkl B Arnholdt HM Jähnig H Spatz H Anthuber M Oruzio DV et al A new concept for the role of ex vivo sentinel lymph nodes in node-negative colorectal cancer. Ann Surg Oncol (2010) 17:2647–55. 10.1245/s10434-010-1030-3
11.
Leiloglou M Kedrzycki MS Chalau V Chiarini N Thiruchelvam PT Hadjiminas DJ et al Indocyanine green fluorescence image processing techniques for breast cancer macroscopic demarcation. Scientific Rep (2022) 12(1):8607. 10.1038/s41598-022-12504-x
12.
Fry DE . The Hawthorne effect revisited. Dis Colon Rectum (2018) 61(1):6–7. PMID: 29219914. 10.1097/DCR.0000000000000928
13.
Edler D Öhrling K Hallström M Karlberg M Ragnhammar P . The number of analyzed lymph nodes–a prognostic factor in colorectal cancer. Acta Oncologica (2007) 46(7):975–81. 10.1080/02841860701203537
14.
Keller DS Berho M Perez RO Wexner SD Chand M . The multidisciplinary management of rectal cancer. Nat Rev Gastroenterol Hepatol (2020) 17(7):414–29. 10.1038/s41575-020-0275-y
15.
Brouwer NP Stijns RC Lemmens VE Nagtegaal ID Beets-Tan RG Fütterer JJ et al Clinical lymph node staging in colorectal cancer; a flip of the coin? Eur J Surg Oncol (2018) 44(8):1241–6. 10.1016/j.ejso.2018.04.008
16.
Rodriguez-Bigas MA Maamoun S Weber TK Penetrante RB Blumenson LE Petrelli NJ . Clinical significance of colorectal cancer: metastases in lymph nodes< 5 mm in size. Ann Surg Oncol (1996) 3:124–30. 10.1007/BF02305790
17.
Suszták N Besznyák I Almási K Bursics A Kelemen D Borowski DW et al Improved accuracy of lymph node staging and long-term survival benefit in colorectal cancer with ex vivo arterial methylene blue infiltration. Pathol Oncol Res (2022) 28:1610742. PMID: 36330051; PMCID: PMC9624224. 10.3389/pore.2022.1610742
18.
Tan L Liu ZL Ma Z He Z Tang LH Liu YL et al Prognostic impact of at least 12 lymph nodes after neoadjuvant therapy in rectal cancer: a meta-analysis. World J Gastrointest Oncol (2020) 12(12):1443–55. PMID: 33362914; PMCID: PMC7739152. 10.4251/wjgo.v12.i12.1443
19.
Lykke J Jess P Roikjaer O Danish Colorectal Cancer Group. Increased lymph node yield is associated with improved survival in rectal cancer irrespective of neoadjuvant treatment: results from a national cohort study. Dis Colon Rectum (2015) 58:823–30. 10.1097/DCR.0000000000000429
20.
Parnaby CN Scott NW Ramsay G MacKay C Samuel L Murray GI et al Prognostic value of lymph node ratio and extramural vascular invasion on survival for patients undergoing curative colon cancer resection. Br J Cancer (2015) 113(2):212–9. Epub 2015 Jun 16. PMID: 26079302; PMCID: PMC4506392. 10.1038/bjc.2015.211
Summary
Keywords
indocyanine green, colorectal cancer, lymph node, ex vivo , colorectal resection
Citation
Lakatos L, Illyes I, Budai A, Bencze V, Szijarto A, Kiss A and Banky B (2024) Feasibility of indocyanine green (ICG) fluorescence in ex vivo pathological dissection of colorectal lymph nodes—a pilot study. Pathol. Oncol. Res. 30:1611853. doi: 10.3389/pore.2024.1611853
Received
29 May 2024
Accepted
19 July 2024
Published
29 August 2024
Volume
30 - 2024
Edited by
Andrea Ladányi, National Institute of Oncology (NIO), Hungary
Updates
Copyright
© 2024 Lakatos, Illyes, Budai, Bencze, Szijarto, Kiss and Banky.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lorand Lakatos, lakatoslorandl@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.