- 1Department of Human Pathology, Graduate School of Medicine, Juntendo University, Tokyo, Japan
- 2Department of Neurosurgery, Graduate School of Medicine, Juntendo University, Tokyo, Japan
Introduction: Mesenchymal chondrosarcoma (MCS) is a rare subtype of chondrosarcoma that occurs at widespread anatomical locations, such as bone, soft tissue, and intracranial sites. The central nervous system (CNS) is one of the most common origins of extraosseous MCS. However, alternative HEY1::NCOA2 fusions have not been reported in this tumor.
Case report: We report a case of intracranial MCS with HEY1::NCOA2 rearrangement. A 52-year-old woman presented with a 15-mm calcified mass around the sella turcica. She initially underwent transsphenoidal surgery for tumor resection and then additional resections for five local recurrences over 5 years. Histologically, the tumor was composed of small round to spindle-shaped cells admixed with well-differentiated hyaline cartilaginous islands. A hemangiopericytoma-like vascular pattern and small sinusoid-like vessels were also observed. RNA sequencing using RNA extracted from formalin-fixed paraffin-embedded samples from the last operation revealed two alternative variants of the HEY1::NCOA2 fusion: HEY1(ex4)::NCOA2 (ex13) and HEY1(ex4)::NCOA2(ex14). Both variants were confirmed as in-frame fusions using reverse transcription-polymerase chain reaction.
Discussion: Cartilaginous components were often not apparent during the recurrences. In addition to the non-typical pathological finding, the correct diagnosis was hampered by the poor RNA quality of the surgical specimens and non-specific STAT6 nuclear staining.
Conclusion: This is the first reported case of intracranial MCS with an alternative HEY1::NCOA2 fusion.
Introduction
Mesenchymal chondrosarcoma (MCS) is a rare subtype of chondrosarcoma, accounting for only 2%–4% of all chondrosarcomas [1]. MCS is distributed in the bone, soft tissue, and intracranial sites. Although the meninges are one of the most common extraskeletal origins of MCS [2], intracranial MCS is an extremely rare tumor of the central nervous system (CNS). Intracranial MCS usually occurs in adolescents and young adults and accounts for less than 1% of all intracranial tumors [3]. When limited to sarcomas, MCS accounts for 11.5% of all CNS sarcomas [4]. One of the most frequent sites for MCSs is the head and neck region (which contains bones in addition to the CNS), accounting for 13% of MCSs [5]. In 2012, HEY1::NCOA2 fusion was identified in MCS [6]. Subsequently, IRF2BP2::CDX1 fusion was detected [7]. In 2020, NKX3.1 expression was reported as a useful immunohistochemical marker for MCS [8, 9]. By identifying such fusion genes or confirming NKX3.1 expression by immunohistochemistry (IHC), it is easier to reach an accurate diagnosis of MCS. MCS is still a very rare tumor, and it goes without saying that listing MCS in the differential diagnoses is important to perform the above diagnostic utilities. We describe a case of intracranial MCS harboring alternative variants of the HEY1::NCOA2 fusion gene in a 52-year-old woman.
Case report
Clinical case
A 52-year-old Japanese woman initially noticed haze in her left eye. She was referred to our hospital because she subsequently showed gradual exacerbation of bitemporal hemianopia. She and her family had no specific medical history. Computed tomography of the head revealed a 15-mm calcified mass in the sella turcica. Magnetic resonance imaging (MRI) of the brain revealed a mass protruding over the sella turcica, with the normal pituitary gland pressed to the upper right and the optic chiasm pressed to the upper left (Figures 1A, B).
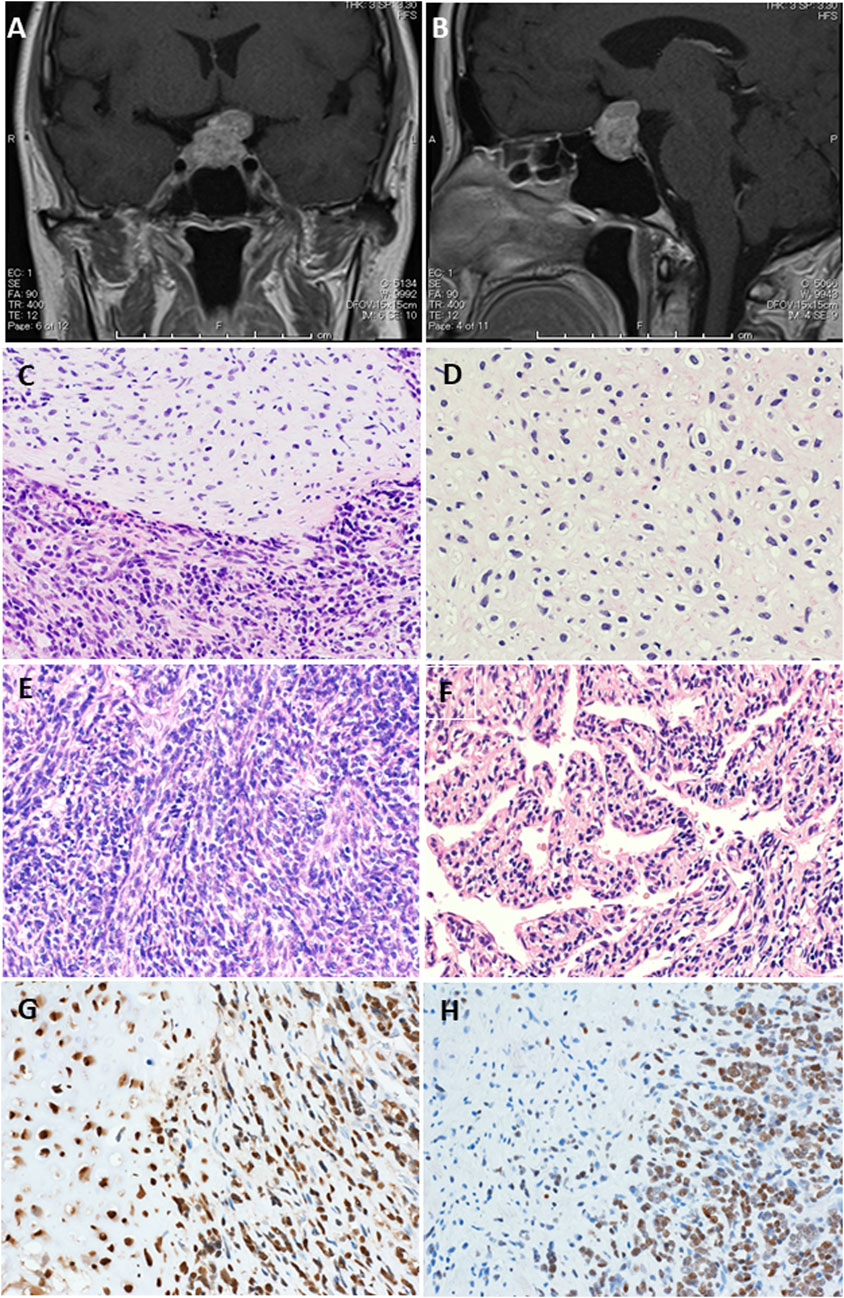
Figure 1. Magnetic resonance imaging (MRI) with contrast enhancement. Preoperative MRI showed a protruding mass above the sella turcica [(A) coronal view, (B) sagittal view]. (C–F) Representative pathological findings. Hematoxylin-eosin staining showed a tumor composed of small round cells and islands of well-differentiated hyaline cartilage with atypical chondroid cells floating in lacunar spaces (original magnification, ×200) (C). A neoplastic cartilage cells do not show higher nuclear atypia, but they have constricted nuclei (original magnification, ×200) (D). Areas of dense proliferation of spindle cells are also encountered (original magnification, ×200) (E). A hemangiopericytomatous proliferation pattern is also noted (original magnification, ×200) (F). SOX9 immunohistochemistry shows positive staining in both spindle cell and cartilaginous areas, whereas NKX3.1 immunohistochemistry shows positive staining almost only in spindle cell area (original magnification, ×200, each) (G).
Surgery
Transsphenoidal surgery was performed, but unfortunately it was incomplete resection. The pathological diagnosis was limited to malignant tumor, with differential diagnoses of solitary fibrous tumor (SFT) and MCS. Ten months later, MRI revealed tumor recurrence. The relapsed tumor was resected via craniotomy, and the patient received stereotactic radiotherapy (SRT). Four years after SRT, she underwent four of surgical resections and one SRT for several local recurrences.
Histopathological findings
Histologically, the tumor was mainly composed of small round cells, and some well-differentiated hyaline cartilage islands with atypical chondroid cells floating in the lacunar spaces were also observed. The cellularity of the atypical chondroid cells was high. No ossification or calcification within the cartilaginous area was observed. In some places, the small cells exhibited a spindle-shaped morphology. A hemangiopericytomatous proliferation pattern was also noted (Figures 1C–F). No meningothelial architecture was observed. Immunohistochemical examinations were performed using antibodies against CD99 (12E7; Dako, Santa Clara, CA, United States), S100 (polyclonal; Dako), STAT6 (YE361; Abcam, Cambridge, United Kingdom), CD34 (QBEnd10; Dako), EMA (E29; Dako), cytokeratin AE1/AE3 (AE1/AE3; Dako), CAM5.2 (CAM5.2; BD Biosciences), SOX9 (EPR14335; Abcam), NKX3.1 (D6D2Z; Cell Signaling), p53 (PAB1801; Thermo Fisher Scientific), and Ki-67 (MIB-1; Dako) following the manufacturer’s instructions. Immunohistochemically, the small round to spindle-shaped cells were positive for CD99 but negative for STAT6, CD34, EMA, and AE1/AE3, whereas the cartilaginous lesions were focally positive for S100. The tumor cells showed focal staining for CAM5.2, but p53 overexpression was not evident. The Ki-67 labeling index was approximately 50%. Then, RNA sequencing (Riken Genesis Co., Ltd., Kanagawa, Japan) was performed using RNA from FFPE samples from the last operation, and two subtypes of HEY1::NCOA2 fusion were identified: fused HEY1 ex4 to NCOA2 ex13 and fused HEY1 ex4 to NCOA2 ex14. RNA sequencing revealed that read counts for HEY1 (ex4)::NCOA2 (ex13) fusion and HEY1 (ex4)::NCOA2 (ex14) fusion were 566 and 172, respectively. Both alterations were confirmed as in-frame fusions by RT-PCR using the following primer pairs: 5′-ACCGGATCAATAACAGTTTG-3′ (HEY1-F1) and 5′-GTGATACCTCAGCCAGGA-3′ (NCOA2-R1) and 5′-CCGAGATCCTGCAGATGACC-3′ (HEY1-F2) and 5′-GCCAAAGACAGACGCTTCAG-3′ (NCOA2-R2) (Figures 2A–C). Finally, this case was diagnosed as mesenchymal chondrosarcoma. Then, additional immunohistochemical examinations were performed, and positive stainings for SOX9 and NKX3.1 were confirmed (Figures 1G, H).
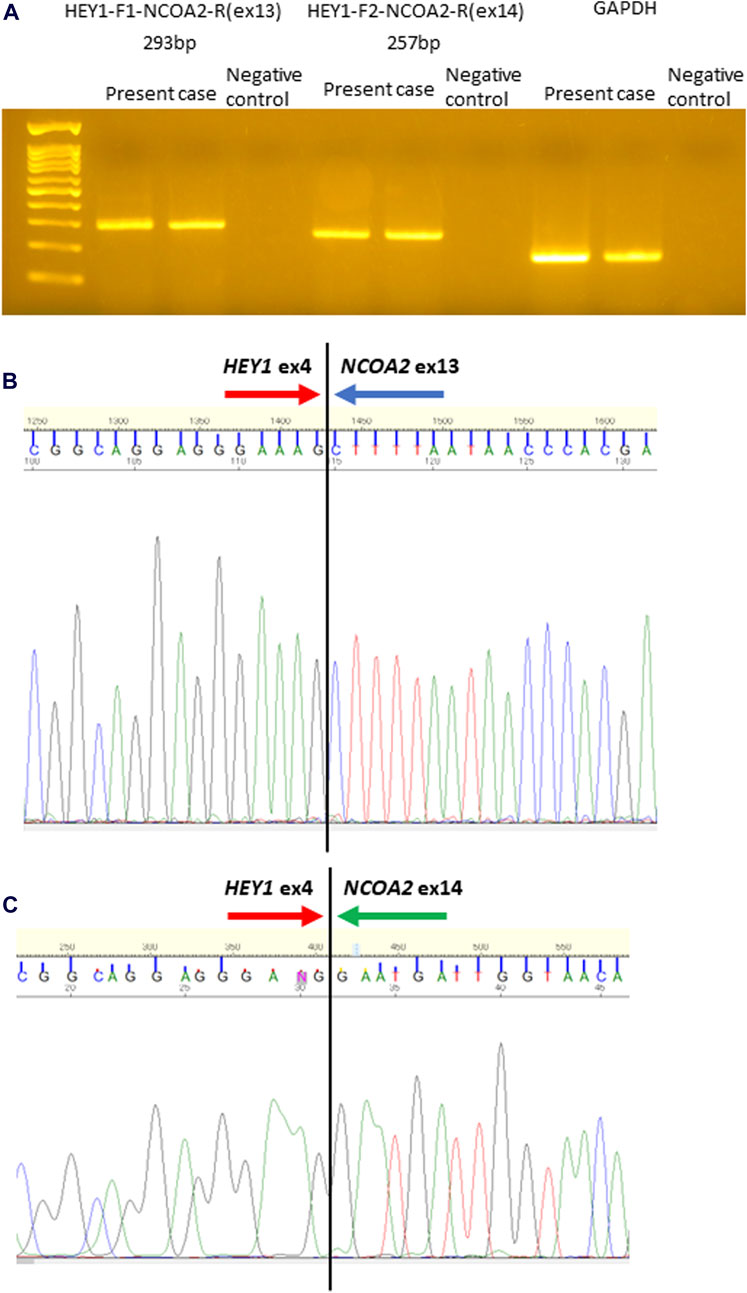
Figure 2. Identification of HEY1::NCOA2 fusion using reverse transcription-polymerase chain reaction (RT-PCR) followed by direct sequencing. RT-PCR was performed using HEY1(ex4)::NCOA2(ex13) fusion- and HEY1(ex4)::NCOA2(ex14) fusion-specific primer pairs. Anticipated sizes for each RT-PCR product were 293 and 257 bp, respectively (A). Sequence analysis demonstrated that exon 4 of HEY1 was fused to exon 13 of NCOA2 (B) and exon 14 of NCOA2 (C).
Follow up
After several times of surgery, the patient experienced blindness and hydrocephalus due to invasion of the recurrent tumors. Regarding the additional therapy to this patient, chemotherapy was not performed, because resistance to chemotherapy and radiation therapy has been reported in conventional chondrosarcoma and it is still controversial in mesenchymal chondrosarcoma [10–13].
Discussion
At the initial resection, characteristic pathological findings of small round cells admixed with well-differentiated hyaline cartilaginous islands suggested MCS as a differential diagnosis. Tumor-specific HEY1::NCOA2 fusion was not detected at that time, probably because of poor sample quality. In this case, we could not confirm the simultaneous presence of cartilaginous components and small round cell areas in several recurrent surgical specimens. In addition, undifferentiated small round cells frequently showed a spindle-shaped morphology with a hemangiopericytomatous vascular pattern. Furthermore, repeated STAT6 IHC revealed no nuclear staining which ruled out the possibility of an SFT.
Clinicopathologic characteristics of MCS in head and neck regions including brain origin was well described in a recent study [11]. It occurs in relatively younger age, and the median age at diagnosis was 19 years (range: 6–54 years) [11]. The patient in this case was oldest among 4 MCS of brain origin in that study [11]. Absence of cartilagenous area was observed in 4 of 13 cases [11] as seen in the recurrent tumor of our case.
Histologically, the tumor was composed of small round to spindle-shaped cells with a hemangiopericytomatous vasculature. Staghorn/hemangiopericytomatous vessels were also described as a frequent histological feature as seen in this case [11]. The differential diagnoses were Ewing’s sarcoma, synovial sarcoma, and SFT. It is difficult to correctly diagnose MCS, especially when only a small round cell area is collected, as was the case for the second and subsequent surgical specimens in this case. Immunostaining for NKX3.1 [8] and SOX9, a master regulator of chondrogenesis, has been reported to be useful [14]. IHC for NKX3.1 and SOX9 was also performed in this case. Tumor cells in both small round/spindle cell and cartilage areas showed positive staining for SOX9, while almost only small round/spindle cell area showed positive staining for NKX3.1. Thus, IHC using these antibodies may be able to distinguish tissues composed of only small round cells without cartilage components.
HEY1(ex4)::NCOA2(ex13) fusion has been reported as HEY1-NCOA2 fusion in MCS; however, HEY1(ex4)::NCOA2(ex14) fusion has not been reported to date. The mechanism of generating the two alternative types of HEY1::NCOA2 fusion in this case [HEY1(ex4)::NCOA2(ex13) fusion and HEY1(ex4)::NCOA2(ex14) fusion] is unknown, although HEY1(ex4)::NCOA2(ex13) fusion was approximately three times dominant than HEY1(ex4)::NCOA2(ex14) fusion. These two forms of HEY1::NCOA2 fusion might be caused by splicing alterations, as an EWSR1::ATF1 fusion was identified in clear cell sarcoma [15], although we do not have any data. Furthermore, it is not clear whether this short form of HEY1::NCOA2 fusion lacking exon13 of NCOA2 is oncogenic, since the functional study has not so far been performed.
Therapeutic strategies that are currently believed to reduce the risk of recurrence include radical resection with radiation and chemotherapy. Xu et al. reported that two patients with MCS who received neoadjuvant chemotherapy did not show any therapeutic response [11], and Huvos et al. also demonstrated that four patients with MCS did not show response to preoperative high dose methotrexate-based chemotherapy [12]. However, Tsuda et al. found stable disease in three MCS patients and partial response in one MCS patient treated with neoadjuvant chemotherapy [13]. Thus, chemotherapeutic effect on MCS is still controversial, and new therapeutic option is expected.
Regarding tumorigenesis, Qi et al. reported that platelet-derived growth factor receptor alpha, which belongs to a family of receptor tyrosine kinases, was upregulated by HEY1::NCOA2 fusion in a study using transduced induced pluripotent stem cell MSCs with inducible expression of the HEY1::NCOA2 fusion protein [16]. In addition, a recent report showed that imatinib, a tyrosine kinase inhibitor (TKI), significantly reduced tumor growth in the HEY1::NCOA2 fusion-driven cellular model as well as in MCS-patient derived xenograft models [17]. Therefore, although further research is required, TKI may be effective in treating MCS.
Conclusion
We encountered a case of intracranial MCS harboring two alternative forms of the HEY1::NCOA2 fusion transcripts.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Juntendo University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SK, TS, and TY performed histopathological and immunohistochemical examinations. AK provided the clinical information on the patient. SK and TS conceived the molecular experiments and analyzed data. TS takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported in part by a Grant-in-Aid from the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Number #20K07415 to TS).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. WHO Classification of Tumours Editorial Board. Soft tissue and bone tumours WHO classification of tumours series, 5th edition, volume 3. Lyon (France): International Agency for Research on Cancer (2020).
2. WHO Classification of Tumours Editorial Board. Central nervous system tumours WHO classification of tumours series, 5th edition, volume 6. Lyon (France): International Agency for Research on Cancer (2021).
3. Bloch, OG, Jian, BJ, Yang, I, Han, SJ, Aranda, D, Ahn, BJ, et al. A systematic review of intracranial chondrosarcoma and survival. J Clin Neurosci (2009) 16(12):1547–51. doi:10.1016/j.jocn.2009.05.003
4. Haider, AS, Palmisciano, P, Sagoo, NS, Bin Alamer, O, El Ahmadieh, TY, Pan, E, et al. Primary central nervous system sarcomas in adults: a systematic review. Clin Neurol Neurosurg (2022) 214:107127. doi:10.1016/j.clineuro.2022.107127
5. Frezza, AM, Cesari, M, Baumhoer, D, Biau, D, Bielack, S, Campanacci, DA, et al. Mesenchymal chondrosarcoma: prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur J Cancer (2015) 51(3):374–81. doi:10.1016/j.ejca.2014.11.007
6. Wang, L, Motoi, T, Khanin, R, Olshen, A, Mertens, F, Bridge, J, et al. Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer (2012) 51(2):127–39. doi:10.1002/gcc.20937
7. Nyquist, KB, Panagopoulos, I, Thorsen, J, Haugom, L, Gorunova, L, Bjerkehagen, B, et al. Whole-transcriptome sequencing identifies novel IRF2BP2-CDX1 fusion gene brought about by translocation t(1;5)(q42;q32) in mesenchymal chondrosarcoma. PLoS One (2012) 7(11):e49705. doi:10.1371/journal.pone.0049705
8. Yoshida, KI, Machado, I, Motoi, T, Parafioriti, A, Lacambra, M, Ichikawa, H, et al. NKX3-1 Is a useful immunohistochemical marker of EWSR1-NFATC2 sarcoma and mesenchymal chondrosarcoma. Am J Surg Pathol (2020) 44(6):719–28. doi:10.1097/PAS.0000000000001441
9. Yoshida, A, Hashimoto, T, Ryo, E, Yoshida, KI, Motoi, T, Yatabe, Y, et al. Confirmation of NKX3-1 expression in EWSR1-NFATC2 sarcoma and mesenchymal chondrosarcoma using monoclonal antibody immunohistochemistry, RT-PCR, and RNA in situ hybridization. Am J Surg Pathol (2021) 45(4):578–82. doi:10.1097/PAS.0000000000001627
10. Tlemsani, C, Larousserie, F, De Percin, S, Audard, V, Hadjadj, D, Chen, J, et al. Biology and management of high-grade chondrosarcoma: an update on targets and treatment options. Int J Mol Sci (2023) 24(2):1361. doi:10.3390/ijms24021361
11. Xu, B, Rooper, LM, Dermawan, JK, Zhang, Y, Suurmeijer, AJH, Dickson, BC, et al. Mesenchymal chondrosarcoma of the head and neck with HEY1::NCOA2 fusion: a clinicopathologic and molecular study of 13 cases with emphasis on diagnostic pitfalls. Genes Chromosomes Cancer (2022) 61(11):670–7. doi:10.1002/gcc.23075
12. Huvos, AG, Rosen, G, Dabska, M, and Marcove, RC. Mesenchymal chondrosarcoma. A clinicopathologic analysis of 35 patients with emphasis on treatment. Cancer (1983) 51(7):1230–7. doi:10.1002/1097-0142(19830401)51:7<1230::aid-cncr2820510710>3.0.co;2-q
13. Tsuda, Y, Ogura, K, Hakozaki, M, Kikuta, K, Ae, K, Tsuchiya, H, et al. Mesenchymal chondrosarcoma: a Japanese musculoskeletal oncology group (JMOG) study on 57 patients. J Surg Oncol (2017) 115(6):760–7. doi:10.1002/jso.24567
14. Wehrli, BM, Huang, W, De Crombrugghe, B, Ayala, AG, and Czerniak, B. Sox9, a master regulator of chondrogenesis, distinguishes mesenchymal chondrosarcoma from other small blue round cell tumors. Hum Pathol (2003) 34(3):263–9. doi:10.1053/hupa.2003.41
15. Wang, WL, Mayordomo, E, Zhang, W, Hernandez, VS, Tuvin, D, Garcia, L, et al. Detection and characterization of EWSR1/ATF1 and EWSR1/CREB1 chimeric transcripts in clear cell sarcoma (melanoma of soft parts). Mod Pathol (2009) 22(9):1201–9. doi:10.1038/modpathol.2009.85
16. Qi, W, Rosikiewicz, W, Yin, Z, Xu, B, Jiang, H, Wan, S, et al. Genomic profiling identifies genes and pathways dysregulated by HEY1-NCOA2 fusion and shines a light on mesenchymal chondrosarcoma tumorigenesis. J Pathol (2022) 257(5):579–92. doi:10.1002/path.5899
Keywords: mesenchymal chondrosarcoma, intracranial mesenchymal chondrosarcoma, CNS sarcoma, HEY1::NCOA2, sella turcica
Citation: Kishikawa S, Kondo A, Yao T and Saito T (2024) Case report: A mesenchymal chondrosarcoma with alternative HEY1::NCOA2 fusions in the sella turcica. Pathol. Oncol. Res. 30:1611730. doi: 10.3389/pore.2024.1611730
Received: 15 February 2024; Accepted: 25 July 2024;
Published: 06 August 2024.
Edited by:
Valeria Barresi, University of Verona, ItalyCopyright © 2024 Kishikawa, Kondo, Yao and Saito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tsuyoshi Saito, dHlzYWl0b3VAanVudGVuZG8uYWMuanA=
 Satsuki Kishikawa
Satsuki Kishikawa Akihide Kondo2
Akihide Kondo2