Abstract
Background:
Peripherally inserted central catheters (PICC) are increasingly used in clinical practice, which also leads to an increased incidence of PICC-related thrombosis. Local thrombus formation could be prevented by limb ischemic preconditioning (IPC). This study aimed to determine whether IPC can prevent deep vein thrombosis in patients with PICC.
Methods:
A total of 600 breast cancer patients receiving PICC were randomized into two groups between July 2016 and July 2018 at the Department of Radiation Oncology. In the preconditioning group, 5 min of ischemic preconditioning was performed three times before PICC, whereas no preconditioning was performed in the sham group. The coagulation function levels, the PICC-related complications, the length of hospital stay, the cost of hospitalization, and the satisfaction of patients were compared.
Results:
The coagulation function levels of patients in the preconditioning group were more normal than in patients from the sham group. In total, 56/300 patients in the sham group had presence of PICC-related thrombosis, with only 23/300 in the IPC group, with no significant difference in other complications between the two groups. However, a longer hospital stay was observed in the sham group compared to the IPC group. Moreover, the cost of hospitalization was also reduced in the IPC group, which also improved the satisfaction of patients.
Conclusion:
Limb ischemic preconditioning may attenuate the severity of vein thrombosis in patients with PICC, which contributes to reducing the incidence of PICC-related thrombosis in clinical practice.
Introduction
Peripherally inserted central catheters (PICCs) are a form of intravenous access in which the catheter is directed from a peripheral vein to a large heart vein. These catheters can be used for an extended period of time and minimize the stimulation of drugs to blood vessels [1–3]. PICC is now increasingly used in clinical practice owing to its safety and cost-effectiveness. However, PICC also involves some complications, such as puncture failure, phlebitis, infection, and thrombosis. PICC-related thrombosis is a severe complication that may lower the quality of life of patients, increase hospitalization costs, and even increase mortality [4, 5]. Patients and doctors are both concerned with PICC-related thrombosis. To reduce the incidence of PICC-related thrombosis, a number of studies have investigated the risk factors and prevention measures for thrombosis in patients with PICC.
Remote ischemic preconditioning (RIPC) is a process by which brief periods of ischemia trigger protective effects on remote organs or tissues. RIPC is widely used in patients to protect organs or tissues prone to ischemic injury, including the liver, brain, myocardium, and so on [6–8]. Research revealed that limb ischemic preconditioning (IPC) could promote thrombus recanalization and reduce the incidence of thrombus in rats [9]. The three main factors necessary for thrombosis formation include stasis of blood, vessel wall injury, and platelet aggregation [10, 11]. Multiple studies have demonstrated that remote IPC before radiofrequency catheter ablation significantly reduced the platelet activation and reactivity of patients with paroxysmal atrial fibrillation [12].
Based on the above content, 600 breast cancer patients receiving PICC in our department were randomized into two groups (sham group and preconditioning group) with 1:1 allocation. Then, the efficacy of catheterization, the coagulation function levels, the incidence of thrombosis, the length of hospital stay, the cost of hospitalization, and the satisfaction of patients were compared between the two groups. The results may verify our hypothesis that limb ischemic preconditioning could play a protective role in the incidence of thrombus after PICC, which could contribute to reducing the incidence of PICC-related thrombosis in clinical practice.
Methods
Experiment design
This single-center, parallel, and double-blind trial included 600 breast cancer patients receiving PICC, who were randomly assigned to two groups with 1:1 allocation. The patients in the preconditioning group were subjected to 5 minutes of ischemic preconditioning three times before PICC, whereas no preconditioning was performed in the sham group. The study was conducted in accordance with the Declaration of Helsinki. The studies involving human participants were reviewed and approved by Xuzhou Central Hospital Biomedical Research Ethics Review Committee (Permit Number: XZXY-LJ-20161210-032). The patients/participants provided their written informed consent to participate in this study.
Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) Adults older than 18 years and younger than 70 years; 2) Patients all underwent mastectomy for breast cancer and needed chemotherapy; 3) Patients were diagnosed with breast cancer with TNM stages II-III; 4) The patients volunteered for PICC, signed the treatment consent form, and volunteered to participate in this project; 5) Normal coagulation function, and 6) No other past Medical History. The exclusion criteria were as follows: 1) Patients older than 70 years or younger than 18 years; 2) Patients who underwent mastectomy for breast cancer but did not require chemotherapy; 3) Patients were diagnosed with breast cancer with TNM stages other than II-III; 4) Patients refused to undergo PICC or refused to enter the project; 5) Abnormal coagulation function, and 6) The presence of other past Medical History.
Surgical procedures
We used the PICC Center venous catheter (BARD, United States), with specifications: Groshong NXT ClearVue 4Fr single-lumen basic kit, including a micro-introducer sheath. The operating procedure for PICC was carried out as described by Amerasekera SS et al. PICC insertion was carried out by the same vascular access team. The success of PICC placement was confirmed based on the color Doppler ultrasound and interventional radiology [4].
Treatment of patients in the sham and IPC group
Both patients in the sham and IPC groups had blood pressure cuffs around their arm while undergoing PICC. The cuff was inflated to 200 mm Hg three times for 5 min in the IPC group and was released to allow reperfusion between the cycles. No preconditioning was performed in patients of the sham group.
Measurement of coagulation function levels
Blood samples of patients were used for coagulation function tests, which were collected from the vena mediana cubiti before PICC and at days 14, 30, 60, and 90 after PICC. The coagulation function test included prothrombin time (PT), thrombin time (TT), activated partial thromboplastin time (APTT), fibrin(-ogen) degradation products (FDP), and D-Dimer. All tests were performed in the clinical laboratory of Xuzhou City Central Hospital.
The observation of PICC-related complications
Local inflammation, bloodstream infection, occlusion, dislocation, PICC-related thrombosis, local bleeding, pulmonary artery embolism, and local allergic reaction were observed and recorded. Patients were subjected to color Doppler ultrasound (SIEMENS, ACUSON S3000) to assess the incidence of thrombosis at days 14, 30, 60, and 90 after PICC.
Statistical analysis
All data are expressed as the mean ± standard deviation. The data were analyzed using variance, q-test, and Student’s t-tests. P < 0.05 was considered statistically significant. SPSS14.0 software was used for all statistical analyses.
Results
Evaluation of PICC
After the catheter insertion, an X-ray was taken to determine the position of the catheter tip. Patient characteristics, procedural details, and the results of X-rays are shown in Figure 1 and Table 1. All patients had no statistically significant differences in baseline information, except for intraoperative blood loss. However, since all patients underwent PICC catheter placement 1 month after surgery, the difference in intraoperative blood loss did not affect their coagulation function and thrombus formation.
FIGURE 1
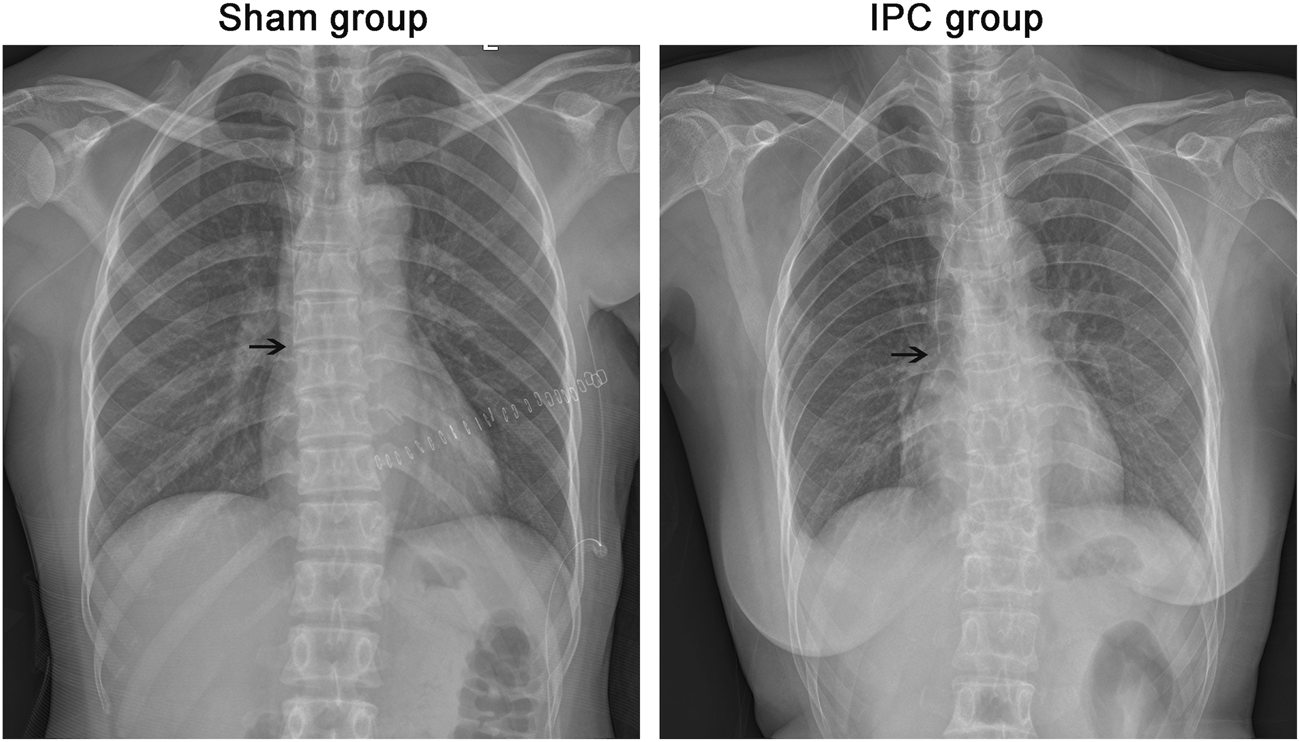
Evaluation of PICC. After the operation, all the cases were taken x-ray to determine the position of the catheter tip. The end of PICC catheter was in normal position between T5 and T7. The black arrow shows the end of the catheter.
TABLE 1
| IPC | Sham | T value | P-value | ||
|---|---|---|---|---|---|
| General data | Age (years) | 53.36 ± 15.61 | 54.31 ± 15.06 | 0.761 | 0.447 |
| BMI | 22.78 ± 3.44 | 22.96 ± 3.58 | 0.629 | 0.53 | |
| X2 value | P-value | ||||
| Right/left breast, n (%) | 147/153, (49/51) | 135/165, (45/55) | 0.96 | 0.33 | |
| Clinical data | Patients undergoing first resection, n (%) | 300, (100) | 298, (99.3) | 2.01 | 0.16 |
| TNM II stage, n (%) | 211, (70.3) | 202, (67.3) | 0.63 | 0.43 | |
| TNM III stage, n (%) | 89, (29.7) | 98, (32.7) | 0.63 | 0.43 | |
| T value | P-value | ||||
| Surgical data | Surgery duration (min) | 104.82 ± 38.27 | 102.44 ± 38.81 | 0.755 | 0.45 |
| Intraoperative blood loss (mL) | 43.97 ± 27.79 | 50.7 ± 27.95 | 2.96 | <0.05* | |
| X2 value | P-value | ||||
| Halsted surgery, n (%) | 17, (5.7) | 18, (6) | 0.03 | 0.86 | |
| Auchincloss surgery, n (%) | 155, (51.7) | 146, (48.7) | 0.54 | 0.46 | |
| Patey surgery, n (%) | 117, (39) | 124, (41.3) | 0.34 | 0.6 | |
| Conserving surgery, n (%) | 11, (3.7) | 12, (4) | 0.05 | 0.83 | |
| Evaluation of PICC | One-time success, n (%) | 300, (100) | 300, (100) | 1 | 1 |
Patient characteristics, procedural details, and the results of X-rays.
*p < 0.05 compared with the sham group.
Serum coagulation function levels
As shown in Table 2, blood samples were collected to evaluate the values of FDP and D-Dimer, and the coagulation function by PT, APTT, TT, and FIB. As shown in Table 2, all of the values were within the normal reference range, but the values of FDP (days 14, 30, 60, and 90) and D-Dimer (day 60) in the sham group were significantly higher than those in the IPC group (FDP: mg/L, sham group, d14: 3.07 ± 1.91, d30: 3.11 ± 1.74, d60: 2.31 ± 1.51, d90: 2.20 ± 1.62, IPC group, d14: 2.53 ± 1.57, d30: 2.54 ± 1.69, d60: 2.01 ± 1.76, d90: 1.96 ± 1.58. D-Dimer: mg/L FEU, sham group, d60: 0.32 ± 0.26, IPC group, d60: 0.26 ± 0.28. p < 0.05). However, the values of PT (days 14, 30, and 90), TT (days 14, 30, 60, and 90), and APTT (days 14, 30, 60, and 90) in the sham group were higher than those in the IPC group (PT: sec, sham group, d14: 9.71 ± 1.40, d30: 9.46 ± 1.28, d90: 10.07 ± 1.48, IPC group, d14: 10.36 ± 1.58, d30: 9.76 ± 1.65, d90: 10.58 ± 1.88. TT: sec, sham group, d14: 16.05 ± 4.11, d30: 15.57 ± 3.99, d60: 16.34 ± 2.77, d90: 17.49 ± 4.23, IPC group, d14: 18.15 ± 3.78, d30: 16.59 ± 4.75, d60: 17.67 ± 3.52, d90: 18.17 ± 3.56. APTT: sec, sham group, d14: 23.41 ± 5.28, d30: 23.92 ± 3.76, d60: 24.97 ± 4.71, d90: 24.49 ± 5.25, IPC group, d14: 24.78 ± 4.79, d30: 25.03 ± 4.52, d60: 26.61 ± 5.04, d90: 26.91 ± 5.39. p < 0.05).
TABLE 2
| IPC | Sham | T value | P-value | ||
|---|---|---|---|---|---|
| PT | Before PICC | 10.31 ± 1.36 | 10.26 ± 1.31 | 0.474 | 0.636 |
| 14 days after PICC | 10.36 ± 1.58 | 9.71 ± 1.40 | 5.292 | <0.001* | |
| 30 days after PICC | 9.76 ± 1.65 | 9.46 ± 1.28 | 2.498 | 0.013* | |
| 60 days after PICC | 10.19 ± 1.92 | 10.00 ± 1.57 | 1.334 | 0.183 | |
| 90 days after PICC | 10.58 ± 1.88 | 10.07 ± 1.48 | 3.660 | <0.001* | |
| TT | Before PICC | 17.93 ± 3.12 | 18.30 ± 3.11 | 1.438 | 0.151 |
| 14 days after PICC | 18.15 ± 3.78 | 16.05 ± 4.11 | 6.505 | <0.001* | |
| 30 days after PICC | 16.59 ± 4.75 | 15.57 ± 3.99 | 2.825 | 0.005* | |
| 60 days after PICC | 17.67 ± 3.52 | 16.34 ± 2.77 | 5.149 | <0.001* | |
| 90 days after PICC | 18.17 ± 3.56 | 17.49 ± 4.23 | 2.138 | 0.033* | |
| APTT | Before PICC | 27.26 ± 4.63 | 26.62 ± 4.56 | 1.704 | 0.089 |
| 14 days after PICC | 24.78 ± 4.79 | 23.41 ± 5.28 | 3.336 | 0.001* | |
| 30 days after PICC | 25.03 ± 4.52 | 23.92 ± 3.76 | 3.249 | 0.001* | |
| 60 days after PICC | 26.61 ± 5.04 | 24.97 ± 4.71 | 4.099 | <0.001* | |
| 90 days after PICC | 26.91 ± 5.39 | 24.49 ± 5.25 | 5.560 | <0.001* | |
| FDP | Before PICC | 1.80 ± 1.08 | 1.91 ± 1.56 | 1.001 | 0.317 |
| 14 days after PICC | 2.53 ± 1.57 | 3.07 ± 1.91 | 3.770 | <0.001* | |
| 30 days after PICC | 2.54 ± 1.69 | 3.11 ± 1.74 | 4.044 | <0.001* | |
| 60 days after PICC | 2.01 ± 1.76 | 2.31 ± 1.51 | 2.236 | 0.026* | |
| 90 days after PICC | 1.96 ± 1.58 | 2.20 ± 1.62 | 1.822 | 0.069* | |
| D-Dimer | Before PICC | 0.28 ± 0.25 | 0.29 ± 0.28 | 0.438 | 0.661 |
| 14 days after PICC | 0.31 ± 0.39 | 0.33 ± 0.31 | 0.707 | 0.480 | |
| 30 days after PICC | 0.36 ± 0.45 | 0.39 ± 0.45 | 0.689 | 0.491 | |
| 60 days after PICC | 0.26 ± 0.28 | 0.32 ± 0.26 | 2.478 | 0.013* | |
| 90 days after PICC | 0.28 ± 0.33 | 0.29 ± 0.33 | 0.344 | 0.731 |
Serum coagulation function levels.
*p < 0.05 compared with the sham group.
The observation of PICC-related complications
PICC-related complications were observed and recorded, and the incidence rate of the thrombosis was assessed by color Doppler ultrasound at days 14, 30, 60, and 90 after PICC. Moreover, the thrombus was observed on grayscale imaging in all 600 patients. The results revealed that 56/300 patients in the sham group had presence of PICC-related thrombosis, with only 23/300 in the IPC group (Figure 2). No significant difference was found in other complications between the two groups (Table 3).
FIGURE 2
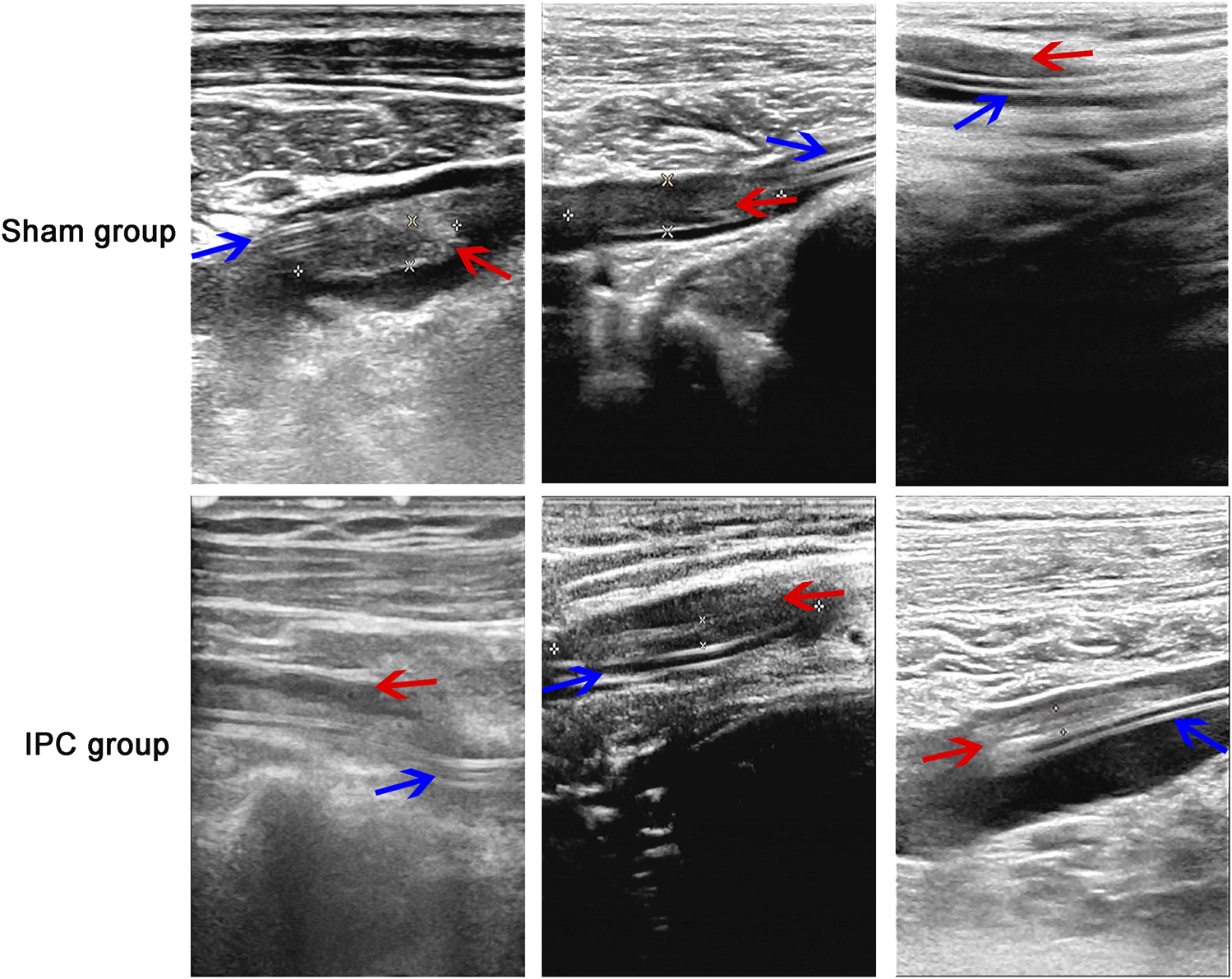
The observation of thrombosis. The absence of PICC-related thrombosis in the sham group was more than that in the IPC group, Substantial hypoechoic and irregular blood flow signals were observed in the vascular lumen of the sham group, and the incidence rate of thrombosis in the IPC group was less than that in the sham group (*p < 0.05 compared with the sham group. Red arrow: thrombosis; blue arrow: catheter).
TABLE 3
| Type of complication | IPC | Sham | X2 value | P-value | |
|---|---|---|---|---|---|
| Local inflammation | n (%) | 21 (7) | 22 (7.3) | 0.025 | 0.87 |
| Bloodstream infection | n (%) | 4 (1.3) | 5 (1.7) | 0.11 | 0.74 |
| Occlusion | n (%) | 3 (1) | 3 (1) | 0 | 1 |
| Dislocation | n (%) | 2 (0.7) | 1 (0.3) | 0.34 | 0.56 |
| PICC-related thrombosis | n (%) | 23 (7.7) | 56 (18.7) | 15.88 | <0.05* |
| Local bleeding | n (%) | 0 (0) | 1 (0.3) | 1 | 0.32 |
| Pulmonary artery embolism | n (%) | 0 (0) | 0 (0) | 0 | 1 |
| Local allergic reaction | n (%) | 1 (0.3) | 1 (0.3) | 0 | 1 |
The observation of related complications.
*p < 0.05 compared with the sham group.
Length of hospital stay
To evaluate the effects of PICC treatment, the length of hospital stay was compared between the two groups. All the 600 individuals were admitted to the hospital 3 times after PICC for chemotherapy. The hospital stays are shown in Table 4, with the hospital stays in the IPC group being shorter than in the sham group [Table 4, hospital stays (day), sham group: 6.77 ± 3.15. IPC group: 6.16 ± 3.03. p < 0.05].
TABLE 4
| IPC | Sham | T value | P-value | |
|---|---|---|---|---|
| Hospital stays(day) | 6.16 ± 3.03 | 6.77 ± 3.15 | 2.15 | <0.05* |
| Hospitalization expenses(Yuan) | 17129.596 ± 2351.66 | 18032.66 ± 1911.64 | 6.83 | <0.05* |
| Patient satisfaction(%) | 95.77 ± 3.66 | 91.9 ± 5.24 | 6.66 | <0.05* |
Hospital stays, hospitalization expenses and patient satisfaction.
*p < 0.05 compared with the sham group.
Hospitalization expenses and patient satisfaction
The cost of hospitalization was also reduced in the IPC group when compared with the sham group, which also improved the satisfaction of patients [Table 4, hospitalization expenses (Yuan), shan group: 18032.66 ± 1911.64, IPC group: 17129.596 ± 2351.66. Patient satisfaction (%), sham group: 91.9 ± 5.24, IPC group: 95.77 ± 3.66. p < 0.05].
Discussion
This study provides useful insights into the effect of limb ischemic preconditioning on the incidence of PICC-related thrombosis in patients with peripherally inserted central catheters. Limb ischemic preconditioning may attenuate the severity of vein thrombosis in patients with peripherally inserted central catheters. The coagulation function levels (PT, TT, APTT, FDP, and D-Dimer) in patients of the sham and IPC groups were first compared, revealing that the values of PT, TT, APTT, FDP, and D-Dimer in patients of the IPC group were normal, but abnormal changes were detected in the coagulation function indicators in the sham group. Then the incidence of PICC-related complications was assessed, displaying a decreased incidence in the IPC group compared with the sham group. The incidence of PICC-related thrombosis in the IPC group was reduced when compared with the sham group, but no significant difference in other complications was observed between the two groups. In addition, the hospital stays were longer in patients of the sham group. Finally, the cost of hospitalization was also lower in the IPC group compared with the sham group, which also improved the satisfaction of patients.
Several approaches to venous access are used in our clinic, including centrally inserted venous catheters as well as peripherally inserted central catheters. When compared with the centrally inserted venous catheters, PICC showed some advantages in the administration of antibiotics, total parenteral nutrition, chemotherapy, fluid replacement, and drug administration. PICC is convenient, easy to operate, safe to insert, and carries a low risk of vascular injury or blood infection. Nevertheless, PICC can also lead to complications, such as infection, phlebitis, and thrombosis. Among these complications, PICC-related thrombosis is relatively common, which may further lead to a potentially serious complication, such as pulmonary embolism. Therefore, proper evaluation and management of PICC are critical for patient health and prognosis, and finding ways to reduce the incidence of catheter-related thrombosis is the main emphasis of our research [13–17].
Ischemic preconditioning was reported to play a protective role in reducing platelet activation, possibly exerting favorable effects on the occurrence of thromboembolic events. Ischemic preconditioning is a process by which the brief periods of ischemia in a tissue protect another organ or tissue. This procedure is widely used and has been verified in our previous study [18–20]. Platelet-derived extracellular vesicles (PEVs), being small particles, extracellular vesicles are associated with various diseases including inflammation, vascular disorders, and tumors. PEVs function similarly to platelets and positively affect hemostasis, thrombus formation, and pro-inflammatory processes through different mechanisms. Additionally, research has discovered that pre-treating limb ischemia can decrease the production of platelet-derived extracellular vesicles which consequently exerts a favorable impact on reducing vascular inflammation and thrombus formation [21]. The current study focused on the process of IPC and thrombus recanalization in patients after PICC. Firstly, PICC was successfully established, and the process was confirmed by an x-ray, which showed the position of the catheter tip. All the patients underwent the PICC procedure successfully.
Coagulation function tests (PT, TT, APTT, FDP, D-dimer, etc.) can effectively evaluate the body’s coagulation status. Research has shown that a hypercoagulable state slows down blood flow and promotes the formation of venous thrombosis. PT, TT, APTT, FDP, D-dimer and other indicators reflect the hypercoagulable state of the body through different mechanisms. For example, TT can indicate the presence of pathological anticoagulant substances in circulating blood; PT is mainly used to reflect the exogenous coagulation pathway; APTT is used to reflect the endogenous coagulation pathway; TT and FDP are primarily used to reflect common coagulation pathways’ status. D-dimer is a specific degradation product produced by fibrin monomers during fibrinolysis. An elevated level of D-dimer reflects secondary fibrinolysis activation. FDP and D-dimer are specific indicators for evaluating high-coagulation states and excessive fibrinolysis in vivo as well as assessing pre-thrombotic hypercoagulability. Therefore, testing coagulation-related indicators at different time points allows assessment of changes in overall coagulation status to evaluate relative risk of thrombus formation [22–24].
Subsequently, the coagulation function was evaluated, revealing that PT, TT, APTT, FDP, and D-Dimer in patients of the IPC group were more normal than that in patients of the sham group. All of the values were within the normal reference range, but the values of FDP (days 14, 30, 60, and 90) and D-Dimer (day 60) in the sham group were significantly higher than those in the IPC group (p < 0.05). However, the values of PT (days 14, 30, and 90), TT (days 14, 30, 60, and 90), and APTT (days 14, 30, 60, and 90) in the sham group were higher than in the IPC group (p > 0.05). Increased PT and APPT values were detected in the sham group after the thrombus was confirmed, which was due to the antithrombotic drugs (such as low molecular weight heparin, warfarin, and so on). Some reports confirmed that IPC could reduce platelet-fibrinogen binding, platelet-neutrophil aggregates, and platelet P-selectin expression, which results in the suppression of platelet activation [12]. All of the above was in accordance with our results.
Secondly, PICC-related complications were observed and recorded. A lower incidence of PICC-related thrombosis was observed in the IPC group compared with the sham group. 56/300 patients in the sham group had presence of PICC-related thrombosis, with only 23/300 in the IPC group. No significant difference in other complications was observed between the two groups, including local inflammation, bloodstream infection, occlusion, dislocation, local bleeding, pulmonary artery embolism, and local allergic reaction. Our results were consistent with the previous study by Ye Sun et al. [25], which suggested that localized lower extremity IPC could reduce deep vein thrombosis formation in a rat experimental thrombosis model. The anti-thrombotic effects of IPC could be attributed to the inhibition of platelet activation [9].
Furthermore, the length of hospital stay was compared between the two groups, showing longer hospital stays in the sham group. The cost of hospitalization was also lower in the IPC group than in the sham group, which also improved the satisfaction of patients.
The mechanism underlying the decreased thrombosis in IPC remains elusive but may involve the release of the antithrombotic substances from the vessel wall, as well as the suppressed effects of IPC on platelets, coagulation, and fibrinolysis. NO (nitric oxide) is a key damaging factor in ischemia/hypoxia, exhibiting dual effects on the body’s protection. During ischemia/hypoxia, it can both improve blood supply to the injured area by dilating local blood vessels and inhibit platelet aggregation and leukocyte adhesion to protect tissues, as well as damage the body by causing DNA injury and generating more toxic oxygen free radicals. NOS is the crucial enzyme for NO synthesis, with three subtypes: neuronal nitric oxide synthase (nNOS), endothelial nitric oxide synthase (eNOS), and inducible nitric oxide synthase (iNOS). When the body experiences ischemia, eNOS activity increases, leading to increased NO production. This causes vasodilation in the ischemic area, improving blood supply and providing protection for ischemic tissues [26, 27]. The protective effects of IPC have yet to be described in animals, and this study further confirmed the action of IPC on thrombosis downregulation in humans. However, the specific experimental mechanism of this protective function requires further animal studies [9, 12, 28–31].
In conclusion, the present study demonstrated that limb ischemic preconditioning may prevent vein thrombosis in patients with peripherally inserted central catheters, which may improve their quality of life and reduce the length of hospitalization. However, the specific mechanism requires further research, and the application of IPC on patients with a high risk of deep venous thrombosis also needs to be analyzed.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Xuzhou Central Hospital Biomedical Research Ethics Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW, HnZ, and CK designed research; YW, HnZ, MH, and CW performed experiments; YW, HnZ, CW, and SZ analyzed data; YW, HnZ, HoZ, QL, MH, and LM interpreted results of experiments; YW, HnZ, HoZ, MH, and LM prepared figures; YW, HnZ, QL, and CK revised manuscript; YW, HnZ, HoZ, QL, and LM approved final version of manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Xuzhou Science and Technology Bureau Foundation (KC22172, KC16SH029) and Xuzhou Municipal Bureau of Science and Technology Key R&D Projects Social Development Projects (KC23178).
Acknowledgments
We thank all patients who voluntarily participated in this study. We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
IPC, Limb ischemic preconditioning; PICC, peripherally inserted central catheter; SOD, superoxide dismutase; NO, nitric oxide.
References
1.
Galen B Baron S Young S Hall A Berger-Spivack L Southern W . Reducing peripherally inserted central catheters and midline catheters by training nurses in ultrasound-guided peripheral intravenous catheter placement. BMJ Qual Saf (2020) 29(3):245–9. Epub 2019 Oct 3. PMID: 31582569. 10.1136/bmjqs-2019-009923
2.
Santos FKY Flumignan RLG Areias LL Sarpe AKP Amaral FCF Ávila RB et al Peripherally inserted central catheter versus central venous catheter for intravenous access: a protocol for systematic review and meta-analysis. Medicine (Baltimore) (2020) 99(30):e20352. PMID: 32791657; PMCID: PMC7386962. 10.1097/MD.0000000000020352
3.
Chen X Liang M . A meta-analysis of incidence of catheter-related bloodstream infection with midline catheters and peripherally inserted central catheters. J Healthc Eng (2022) 12:6383777. PMID: 35313516; PMCID: PMC8934223. 10.1155/2022/6383777
4.
Swaminathan L Flanders S Horowitz J Zhang Q O'Malley M Chopra V . Safety and outcomes of midline catheters vs peripherally inserted central catheters for patients with short-term indications: a multicenter study. JAMA Intern Med (2022) 182(1):50–8. PMID: 34842905; PMCID: PMC8630646. 10.1001/jamainternmed.2021.6844
5.
Mielke D Wittig A Teichgräber U . Peripherally inserted central venous catheter (PICC) in outpatient and inpatient oncological treatment. Support Care Cancer (2020) 28(10):4753–60. Epub 2020 Jan 22. PMID: 31970514; PMCID: PMC7447660. 10.1007/s00520-019-05276-0
6.
Lamidi S Baker DM Wilson MJ Lee MJ . Remote ischemic preconditioning in non-cardiac surgery: a systematic review and meta-analysis. J Surg Res (2021) 261:261–73. Epub 2021 Jan 15. PMID: 33460972. 10.1016/j.jss.2020.12.037
7.
Leurcharusmee P Sawaddiruk P Punjasawadwong Y Sugandhavesa N Klunklin K Tongprasert S et al Ischemic preconditioning upregulates Mitofusin2 and preserves muscle strength in tourniquet-induced ischemia/reperfusion. J Orthop Translat (2022) 35:113–21. PMID: 36312592; PMCID: PMC9582561. 10.1016/j.jot.2022.09.012
8.
Wu J Yu C Zeng X Sun C . The hepatoprotective effect from ischemia-reperfusion injury of remote ischemic preconditioning in the liver related surgery: a meta-analysis. ANZ J Surg (2022) 92(6):1332–7. Epub 2021 Dec 2. PMID: 34854193. 10.1111/ans.17236
9.
Gorog DA Farag M Spinthakis N Yellon DM Bøtker HE Kharbanda RK et al Effect of remote ischaemic conditioning on platelet reactivity and endogenous fibrinolysis in ST-elevation myocardial infarction: a substudy of the CONDI-2/ERIC-PPCI randomized controlled trial. Cardiovasc Res (2021) 117(2):623–34. PMID: 32163139; PMCID: PMC7820881. 10.1093/cvr/cvaa061
10.
Kleidon TM Horowitz J Rickard CM Ullman AJ Marsh N Schults J et al Peripherally inserted central catheter thrombosis after placement via electrocardiography vs traditional methods. Am J Med (2021) 134(2):e79–e88. Epub 2020 Jul 14. PMID: 32673624. 10.1016/j.amjmed.2020.06.010
11.
Yuen HLA Tran H Chunilal S . Upper extremity deep vein thrombosis: current knowledge and future directions. Semin Thromb Hemost (2021) 47(6):677–91. Epub 2021 May 10. PMID: 33971684. 10.1055/s-0041-1725116
12.
Krag AE Kiil BJ Hvas CL Hvas AM . Effect of remote ischemic preconditioning on hemostasis and fibrinolysis in head and neck cancer surgery: a randomized controlled trial. PLoS One (2019) 14(7):e0219496. PMID: 31283796; PMCID: PMC6613699. 10.1371/journal.pone.0219496
13.
Urtecho M Torres Roldan VD Nayfeh T Espinoza Suarez NR Ranganath N Sampathkumar P et al Comparing complication rates of midline catheter vs peripherally inserted central catheter. A systematic review and meta-analysis. Open Forum Infect Dis (2023) 10(2):ofad024. PMID: 36751645; PMCID: PMC9898877. 10.1093/ofid/ofad024
14.
Estrada-Orozco K Cantor-Cruz F Larrotta-Castillo D Díaz-Ríos S Ruiz-Cardozo MA . Central venous catheter insertion and maintenance: evidence-based clinical recommendations. Rev Colomb Obstet Ginecol (2020) 71(2):115–62. PMID: 32770871. 10.18597/rcog.3413
15.
Balsorano P Virgili G Villa G Pittiruti M Romagnoli S De Gaudio AR et al Peripherally inserted central catheter-related thrombosis rate in modern vascular access era-when insertion technique matters: a systematic review and meta-analysis. J Vasc Access (2020) 21(1):45–54. Epub 2019 Jun 10. PMID: 31177939. 10.1177/1129729819852203
16.
Marsh N Webster J Ullman AJ Mihala G Cooke M Chopra V et al Peripheral intravenous catheter non-infectious complications in adults: a systematic review and meta-analysis. J Adv Nurs (2020) 76(12):3346–62. Epub 2020 Oct 5. PMID: 33016412. 10.1111/jan.14565
17.
Hu Q Su Y Yan L . Effects of peripherally inserted central catheter (PICC) catheterization nursing on bloodstream infection in peripheral central venous catheters in lung cancer: a single-center, retrospective study. Comput Math Methods Med (2022) 2022:2791464. PMID: 36158127; PMCID: PMC9499753. 10.1155/2022/2791464
18.
Liang J Han R Zhou B . Metabolic reprogramming: strategy for ischemic stroke treatment by ischemic preconditioning. Biology (Basel) (2021) 10(5):424. PMID: 34064579; PMCID: PMC8151271. 10.3390/biology10050424
19.
Donato M Bin EP D Annunzio V Gelpi RJ . Myocardial remote ischemic preconditioning: from cell biology to clinical application. Mol Cel Biochem (2021) 476(10):3857–67. Epub 2021 Jun 14. PMID: 34125317. 10.1007/s11010-021-04192-4
20.
Litrico L Aid R Youkharibache A Letourneur D Cristofari S . Effect of ischemic preconditioning on skeletal tissue tolerance after warm venous ischemia. Ann Chir Plast Esthet (2023) 68(4):315–25. Epub 2023 Mar 23. PMID: 36966096. 10.1016/j.anplas.2023.02.003
21.
Reddel CJ Pennings GJ Lau JK Chen VM Kritharides L . Circulating platelet-derived extracellular vesicles are decreased after remote ischemic preconditioning in patients with coronary disease: a randomized controlled trial. J Thromb Haemost (2021) 19(10):2605–11. Epub 2021 Aug 10. PMID: 34196106. 10.1111/jth.15441
22.
Wang W Wang JN Yu W Zhu SN Gao Y Zhang JQ . Comparison of coagulation function between adrenocorticotropic hormone independent Cushing syndrome and nonfunctional adrenal adenoma and its influence factors. Beijing Da Xue Xue Bao Yi Xue Ban (2023) 55(6):1062–7. Chinese. PMID: 38101790; PMCID: PMC10723997. 10.19723/j.issn.1671-167X.2023.06.017
23.
Li X Li T Fan Y . Efficacy of intravascular mechanical thrombectomy combined with thrombolysis and anticoagulant therapy in the treatment of cerebral venous sinus thrombosis and its effect on neurological function and coagulation indices. Am J Transl Res (2021) 13(6):6921–8. PMID: 34306444; PMCID: PMC8290642.
24.
M SM Samal DB Amirtraj JV Subramanian S Venkatasubbu GD . Enhanced coagulation cascade activation and styptic effects of Zn@SiO2nanocomposite. Colloids Surf B Biointerfaces (2024) 239:113927. Epub 2024 Apr 25. PMID: 38714078. 10.1016/j.colsurfb.2024.113927
25.
Sun Y Mao P Lu J Li L Lu W Jiang Q et al Localized lower extremity ischemic preconditioning prevents against local thrombus formation. Vasa (2015) 44(4):285–8. PMID: 26314360. 10.1024/0301-1526/a000443
26.
Yusof M Kamada K Kalogeris T Gaskin FS Korthuis RJ . Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO- and p38 MAPK-dependent mechanism. Am J Physiol Heart Circ Physiol (2009) 296(3):H868–76. Epub 2009 Jan 23. PMID: 19168723; PMCID: PMC2660238. 10.1152/ajpheart.01111.2007
27.
Kram L Grambow E Mueller-Graf F Sorg H Vollmar B . The anti-thrombotic effect of hydrogen sulfide is partly mediated by an upregulation of nitric oxide synthases. Thromb Res (2013) 132(2):e112–7. Epub 2013 Aug 2. PMID: 23916820. 10.1016/j.thromres.2013.07.010
28.
Ekeloef S Koyuncu S Holst-Knudsen J Gundel O Meyhoff CS Homilius M et al Cardiovascular events in patients undergoing hip fracture surgery treated with remote ischaemic preconditioning: 1-year follow-up of a randomised clinical trial. Anaesthesia (2021) 76(8):1042–50. Epub 2021 Jan 13. PMID: 33440017. 10.1111/anae.15357
29.
Fu J Cai W Zeng B He L Bao L Lin Z et al Development and validation of a predictive model for peripherally inserted central catheter-related thrombosis in breast cancer patients based on artificial neural network: a prospective cohort study. Int J Nurs Stud (2022) 135:104341. Epub 2022 Aug 8. PMID: 36084529. 10.1016/j.ijnurstu.2022.104341
30.
Matysiak K Szewczuk M Sobocki J Zdziarska M Siatkowski I . Complications of tunneled peripherally inserted and tunneled-cuffed central catheters in home parenteral nutrition. Nutrition (2021) 91-92:111354–92. Epub 2021 May 26. PMID: 34246088. 10.1016/j.nut.2021.111354
31.
Chen P Wan G Zhu B . Incidence and risk factors of symptomatic thrombosis related to peripherally inserted central catheter in patients with lung cancer. J Adv Nurs (2021) 77(3):1284–92. Epub 2020 Nov 29. PMID: 33249623. 10.1111/jan.14666
Summary
Keywords
tumor, limb ischemic preconditioning, peripherally inserted central catheter, vein thrombosis, PICC-related complications
Citation
Zhao H, Kou C, Zhao H, Liu Q, He M, Wang C, Zhu S, Ma L and Wang Y (2024) Impact of limb ischemic preconditioning on the incidence of vein thrombosis in patients with peripherally inserted central catheter. Pathol. Oncol. Res. 30:1611596. doi: 10.3389/pore.2024.1611596
Received
19 November 2023
Accepted
04 November 2024
Published
14 November 2024
Volume
30 - 2024
Edited by
Tamás Micsik, Semmelweis University, Hungary
Updates
Copyright
© 2024 Zhao, Kou, Zhao, Liu, He, Wang, Zhu, Ma and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Wang, wyhh666@gmail.com; Li Ma, 675694863@qq.com
‡These authors have contributed equally to this work
ORCID: Yun wang, orcid.org/0000-0002-5268-4833
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.