- 1Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China
- 2Department of Obstetrics and Gynecology, The Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China, Changsha, China
Background: Extraocular sebaceous carcinoma (SC) arising in the vulva is extremely rare that no treatment consensus has been well-defined.
Case presentation: We here presented two cases of vulval SC in a 31-year-old and a 62-year-old woman, respectively. Radical wide local excision was performed with free margin and they received no postoperative adjuvant therapy. No evidence of disease was detected after follow-ups for 12 months and 49 months, respectively. A comprehensive literature review of vulval SC was further conducted and other ten cases were included. The mean age was 55.9 years, nine patients were diagnosed with FIGO stage I diseases while the remaining three patients had metastatic lesions at initial diagnosis. Surgery was the mainstay treatment option that 11 (91.7%) underwent surgical resection, of which 5 patients received inguinal lymphadenectomy and 2 patients showed lymph nodes involved. Radiotherapy and chemotherapy were given in 2 and 1 patient, respectively. Two patients experienced recurrence within 1 year after initial therapy. At the final follow-up, ten patients had no evidence of disease, one patient was alive with the disease, and only one died of the disease.
Conclusion: Radical wide local excision may be preferred in early-stage vulval SC and utilization of sentinel lymph node sampling should be recommended. Postoperative adjuvant therapy may be spared in patients with negative surgical margin and absence of lymph node involvement. Treatment of vulval SC referring to the guidelines of vulvar cancer should be administered in case of positive margins or metastatic disease.
Introduction
Sebaceous carcinoma (SC) is an uncommon cutaneous malignant tumor with aggressive potential, accounting for less than 5% of all cutaneous malignancies [1]. SC can be divided into two subtypes, periocular SC and extraocular SC, in which about one-third to up to 75% of the SC are classified as periocular SC [2]. Furthermore, over 90% of the extraocular SC locates in the head/neck region, and only 7.2% originates from other sites [3].
Vulval SC is an extremely rare type of extraocular SC and only a handful of cases have been reported [4]. Characterization of the management of this rarity is completely accumulated through several case reports, and treatment strategies have varied greatly, with some researchers applying radical vulvectomy while others chose wide local excision, and some merely administrated excisional biopsy as primary surgical treatment [4–8]. The necessity of inguinal lymphadenectomy is still controversial [2, 4]. Postoperative chemotherapy and radiotherapy have also been reported but the benefit remained unclear [5, 9, 10]. However, currently, the studies only present narrative descriptive survival outcomes, the detailed information on clinical characteristics and prognosis, especially prognostic predictors, needs to be explored in greater depth.
To investigate the clinical characteristics and outcomes of patients with vulval SC, a retrospective study of 12 patients was conducted, including two patients diagnosed in Changsha Central Hospital and 10 cases reviewed in published research. The recurrence-free survival (RFS) and disease-specific survival (DSS) of these patients were further evaluated.
Materials and methods
Our two-step study was approved by the Institutional Review Board of the Changsha Central Hospital. Firstly, we performed a pathological review in the database of our hospital and two cases of vulval SC treated in our hospital were identified. Secondly, in order to enroll all the suitable vulval SC patients reported between 1970 and 2022, we used the following keywords to search in major medical databases (PubMed, Embase, Web of Science, and Scopus): “vulval sebaceous carcinoma”; “sebaceous carcinoma of the vulva”; “primary vulval sebaceous carcinoma”; “sebaceous carcinoma of female genital tract”; “extraocular sebaceous carcinoma.” All the related articles of vulval SC cited by the screened papers would have been also evaluated to determine whether they were eligible. Patients who were diagnosed as extraocular sebaceous carcinoma of other sites or secondary vulval sebaceous carcinoma were excluded from final analysis. Similarly, those who lacked either detailed clinical characteristics or survival outcomes were also eliminated. To ensure the scientific nature and reliability, we excluded vulval SC patients from letter to editors and non-English publications. Moreover, unrelated articles, including imaging studies and or pathological investigations of vulval SC were not subjected to analysis. The Supplementary Figure S1 shows the screening process of our research. We eventually included 12 patients with vulval SC, including 10 patients identified in literature review and the two patients treated in our hospital.
After generation of the database, we summarized and analyzed the patients’ clinical characteristics, treatment details, and survival outcomes. In this study, we defined the interval between the date of initial treatment and the confirmation of disease relapse as recurrence-free survival (RFS). Disease-specific survival (DSS) was calculated from the date of diagnosis establishment up to the death caused by tumor or the last follow-up.
Statistical analysis
Variables descriptions in this study were determined by their distributions. The survival probability was calculated according to the Kaplan-Meier method and the log-rank test. All the statistical analyses were performed by the SPSS (version 27.0; IBM SPSS, Armonk, NY, USA) software.
Results
Case presentation
A total of 52 patients with sebaceous carcinoma were included in the screening and 30 of them were female. In this cohort, periocular sebaceous carcinoma (12 cases) was the most predominant subtype, followed by breast (7 cases), scalp (4 cases), nose (3 cases), parotid gland and inguinal region (1 case, each), in descending order. Furthermore, only two patients were diagnosed with sebaceous carcinoma of the vulva, accounting for 6.7% of this female population.
Case 1
A 62-year-old woman visited the local hospital due to vulval erythema with erosion and itching for 3 years. A vulval skin biopsy was then performed and pathology could not exclude Paget’s disease. Subsequently, an inter-institutional pathological consultation diagnosed carcinoma of the skin appendage, tending to originate from the sebaceous gland. Further whole-body PET/CT did not reveal lesions in other sites and no enlarged lymph nodes were observed. The immunohistochemical (IHC) staining showed partial expression of AR and CK8/18, CK7 and P63 were also positive, with a Ki-67 proliferative index of approximately 70%. No elevated tumor markers or specific comorbidity was noted. Physical examination showed multiple erythemas with the largest diameter of about 2.5 cm on the surface of the left labium and mons pubis, and the distance to the middle line was over 2 cm.
Radical wide local excision with a surgical margin of 2 cm and deep to the fascia was performed (Supplementary Figure S2). The final pathology confirmed the diagnosis of sebaceous carcinoma arising in the vulva. The IHC staining showed positive expression of CD15, CD10, P40, and P63, the Ki-67 proliferative index was 70% (Figure 1). The tumor-infiltrating depth was 1 mm without margin involved (>1 cm), and FIGO stage IB was established based on the pathology and preoperative imaging.
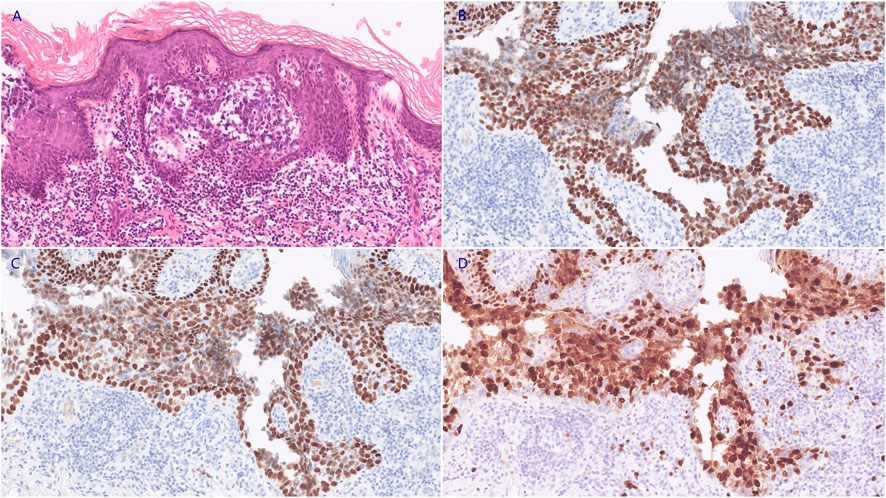
FIGURE 1. The neoplastic cells demonstrated marked pleomorphism, with increased nucleo-cytoplasmic ratio and irregular nucleus shape ((A), HE staining, 200X). The IHC staining revealed positive expression of P40 ((B), 200X) and P63 ((C), 200X). The Ki-67 proliferative index was approximately 70% ((D), 200X).
The postoperative recovery was uneventful and she was discharged 4 days after the surgery. No adjuvant chemotherapy or radiotherapy was administrated. She was requested to follow up in a 3 months interval and showed no evidence of disease 12 months after the surgery.
Case 2
A 31-year-old woman presented a 0.5-cm mild painful nodule with slight itching of the left labium major for nearly 2 years. An excisional biopsy confirmed the diagnosis of moderately differentiated vulval SC (Figure 2). The surgical margin was involved by the tumor and subsequent whole-body PET/CT revealed no metastatic lesion. She was referred to our hospital after the diagnosis had been established. Radical wide local excision was performed. Similarly, we set the surgical margin of 2 cm and deep margin to the fascia, aiming to achieve a pathological margin of at least 8 mm. Postoperatively, pathology revealed no residual tumor, and no further adjuvant therapy was administrated. She remains free of disease at 49 months after treatment.
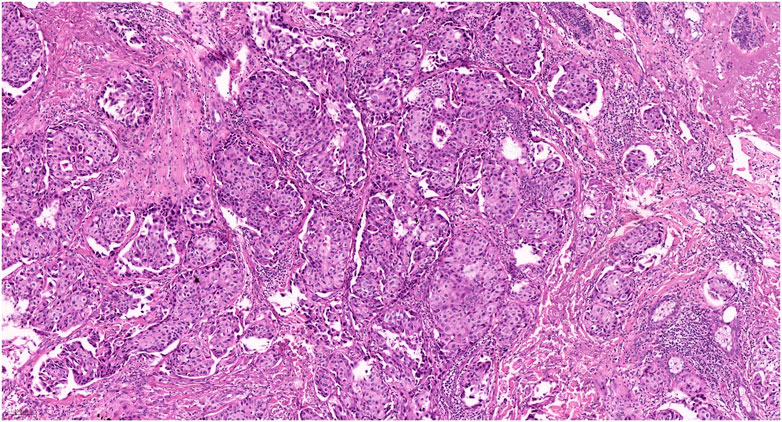
FIGURE 2. Pathology of the second patient revealed classic morphology of sebaceous carcinoma (HE staining, 100X).
Literature review
Twelve patients were included after screening, and the mean age was 55.9 years, only three were younger than 45 years. The mean gross mass size was 1.7 cm in these patients. Most of the primary lesions were in the labia. At the time of initial diagnosis of vulval SC, lesions in 9 patients were classified as FIGO stage I, while metastatic disease was found in three cases, of which two had inguinofemoral lymph nodes involved and one had retroperitoneal lymph nodes and lung metastasis.
The manifestations of vulvar SC were unspecified and may be asymptomatic (Table 1). All patients underwent surgical treatment after diagnosis, except one patient who had lung metastasis. However, the surgical options varied, and excisional biopsy, local excision, wide local excision, radical wide local excision, and radical hemivulvectomy had been used variably. Furthermore, five patients also received lymphadenectomy and two cases showed nodal involvement. Most of these patients (9/11, 81.8%) did not receive any postoperative adjuvant therapy. Radiotherapy was administrated in the two patients who had inguinofemoral lymph node metastasis. Besides, the patient with lung metastasis received platinum-based chemotherapy combined with everolimus but had a poor response.
The median follow-up time was 1.3 years (range: 0.7–13.5 years). During the follow-up, two patients experienced recurrence within 1 year after initial therapy, among them one was diagnosed as FIGO stage III (bilateral inguinal lymph nodes metastasis). These two patients underwent surgical excision but both presented a second recurrence. One of them received palliative chemotherapy due to multiple metastatic lesions. Another patient received a partial vulvectomy that achieved no evidence of disease. At the final follow-up, ten patients achieved no evidence of disease, one patient was alive with the disease, and one died of the disease. The 5-year DSS rates was 91.7%, and the 5-year RFS rate was 81.8%. The median RFS and DSS had not been achieved, with a mean RFS and DSS of 11.1 years (8.1–14.1, 95% confidence interval [CI]) and 12.4 years (10.4–14.4, 95% CI), respectively.
Discussion and conclusion
Our study presents two rare cases of vulval SC and provides a literature review of published cases of vulval SC focusing on clinical characteristics and survival outcomes in this unique population. Patients with vulval SC usually manifested lesions that resemble other vulval cancers of common types without distinct symptoms, and the survival outcome was satisfactory.
The mainstay therapeutic option of extraocular SC remains surgery with complete circumferential peripheral and deep margin assessment to guarantee adequate margins [2]. Rocha et al. [8] reported a patient who experienced two times recurrences due to a surgical margin of less than 1 mm, after a partial vulvectomy with enough margin she achieved no evidence of disease for 1 year. Indeed, the NCCN guidelines recommend re-excision of positive margins or those classified as close (<8 mm) [11]. Our patients are alive without disease relapse after radical wide local excision with a 2-cm margin. Moreover, our study showed 10 of 12 patients showed no evidence of disease at last follow-up, indicating a favorable survival outcome in patients with vulval SC. Furthermore, radical vulvectomy inevitably substantially increases the psychosexual morbidity of the treatment. We herein suggest that radical wide local excision with adequate peripheral and deep margins may be preferred in patients with early-stage vulval SC.
The necessity of inguinal lymphadenectomy has not been well defined. Our study showed a relatively high lymph node involvement rate of 40% in five patients who underwent inguinal lymphadenectomy. Besides, if we include another case reported in a letter [12], this rate goes up to 50% (3 of 6 cases), and whether the lymph node involved or not seemed to have no relation with tumor size, as even a patient with only 5 mm lesion was presented lymph node metastasis. It might be hypothesized that the size of the carcinoma does not equal the lesion site. Nonetheless, only 10 (0.9%) patients had positive lymph nodes in 1,070 cases of extraocular SC [13]. It is unclear why the vulval SC presents a considerably high regional lymph node involvement rate, maybe due to the profuse lymph circulation of the perineum and the location at the vulva may be potentially an intrinsic risk factor for tumor metastasis. Furthermore, the relation between disease-specific mortality of vulval SC and regional lymph node involvement is unknown. To avoid the omitting of positive lymph node excision and reduce the side effect of lymphadenectomy, sentinel lymph node (SLN) sampling has been increasing utilized in vulval SC and extraocular SC [2, 4]. In the GROINSS-V study, the recurrence rate was only 2.3% in 403 patients with negative SLN sampling of vulvar carcinomas, with a 3-year DSS of 97% and remarkably reduced surgical morbidity [14]. Hence, comprehensive imaging, preoperative evaluation and routine SLN sampling in early stage vulval SC may be practical. However, in cases of macrometastases in SLN, bilateral inguinal lymphadenectomy should be conducted since inguinofemoral radiotherapy without surgical resection had a significantly higher recurrence rate [15].
The utility of postoperative adjuvant therapy remains controversial. Radiotherapy may be a candidate for monotherapy for extraocular SC patients who are medically inoperable or have surgically unresectable tumors, positive margin, or nodal metastases with a recommended dose of 50–70 Gy [1, 2]. The GROINSS-V-II trial found that inguinofemoral radiotherapy is a safe alternative for inguinofemoral lymphadenectomy in patients with sentinel node micrometastases [15]. Three patients underwent surgery with radiotherapy due to inguinal lymph node metastasis, all of them were alive and two of them were disease-free [5, 9, 12]. Therefore, postoperative radiotherapy or chemotherapy referenced in guidelines of extraocular SC or vulval cancer may be reasonable. Nonetheless, the only death related to vulval SC in our cohort that received platinum-based chemotherapy due to lung metastasis showed a poor response and succumbed to the disease only 8 months after diagnosis [10].
It should be emphasized that screening of Muir-Torre syndrome (MTS) in patients with vulval SC must also be performed. MTS is characterized by the presence of sebaceous neoplasms and one or more visceral malignancies [16]. The sebaceous tumors include solitary or multiple sebaceous adenoma and/or carcinoma, and colorectal cancer and endometrial cancer are the two most common visceral malignant tumors [17]. Moreover, some MTS patients also have germline mutations in DNA mismatch repair genes MLH1 or MSH2, and are considered a subtype of Lynch syndrome [18]. In our study, both patients had PET/CT to screen for other lesions and coexisting tumors. Although the incidence of MTS is extremely rare [19], realizing the probability in vulval SC patients may allow appropriate screening for visceral malignant tumors to reduce morbidity and mortality.
To sum up, the survival outcomes in patients with vulval SC were favorable with a 5-year RFS rate of 81.8% and a 5-year DSS rate of 91.7%, respectively. Radical wide local excision may be preferred in early-stage vulval SC and utilization of SLN sampling should be recommended. Postoperative adjuvant therapy may be spared in patients with negative surgical margin and absence of lymph node involvement. Treatment of vulval SC referring to the guidelines of vulvar cancer should be administered in case of positive margins or metastatic diseases. Moreover, screening of MTS in patients with vulval SC should be emphasized, especially in young patients. However, due to the extremely rarity of vulval SC, further research is warranted.
Data availability statement
All data generated or analyzed during this study are included in this published article and the Supplementary Information Files. The datasets used and/or analyzed during the current study can be obtained from the corresponding author upon reasonable request.
Ethics statement
This retrospective study was approved by the Ethics Committee of Changsha Central Hospital. Written informed consent to participate in this study and publication of their clinical details and/or clinical images was obtained from the patients.
Author contributions
XXW wrote the manuscript, conducted literature review, and statistical analysis. XW conceived the study design and modified the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Scientific Research Project of Hunan Provincial Health Commission (No. 202205012844) and the Natural Science Foundation of Hunan Province China (No. 2023JJ40073).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2023.1611259/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | The screening details of literature review in our study.
SUPPLEMENTARY FIGURE S2 | The surgical excision specimen of case 1 (suspicious lesions were marked with suture).
References
1. Knackstedt, T, and Samie, FH. Sebaceous carcinoma: A review of the scientific literature. Curr Treat Options Oncol (2017) 18(8):47. doi:10.1007/s11864-017-0490-0
2. Owen, JL, Kibbi, N, Worley, B, Kelm, RC, Wang, JV, Barker, CA, et al. Sebaceous carcinoma: Evidence-based clinical practice guidelines. Lancet Oncol (2019) 20(12):e699–e714. doi:10.1016/S1470-2045(19)30673-4
3. Tripathi, R, Chen, Z, Li, L, and Bordeaux, JS. Incidence and survival of sebaceous carcinoma in the United States. J Am Acad Dermatol (2016) 75(6):1210–5. doi:10.1016/j.jaad.2016.07.046
4. Yamamoto, A, Chigusa, Y, Fujimoto, M, Yamanoi, K, Minamiguchi, S, Yasuda, E, et al. Sebaceous carcinoma of the vulva treated with sentinel lymph node biopsy: A case report and literature review. Int Cancer Conf J (2021) 10(3):239–43. doi:10.1007/s13691-021-00488-w
5. Kawamoto, M, Fukuda, Y, Kamoi, S, Sugisaki, Y, and Yamanaka, N. Sebaceous carcinoma of the vulva. Pathol Int (1995) 45(10):767–73. doi:10.1111/j.1440-1827.1995.tb03395.x
6. Carlson, JW, McGlennen, RC, Gomez, R, Longbella, C, Carter, J, and Carson, LF. Sebaceous carcinoma of the vulva: A case report and review of the literature. Gynecol Oncol (1996) 60(3):489–91. doi:10.1006/gyno.1996.0079
7. Sullivan, SA, Tran, AQ, O'Connor, S, and Gehrig, PA. Sebaceous carcinoma of the vulva: A case report and review of the literature. Gynecol Oncol Rep (2016) 18:40–1. doi:10.1016/j.gore.2016.10.008
8. Rocha, AC, Sá, MI, Abrantes, C, and Sousa, R. Sebaceous carcinoma of the vulva: An unexpected diagnosis and literature review. Acta Med Port (2022) 35(1):63–7. doi:10.20344/amp.13551
9. Khan, Z, Misra, G, Fiander, AN, and Dallimore, NS. Sebaceous carcinoma of the vulva. Bjog (2003) 110(2):227–8. doi:10.1046/j.1471-0528.2003.01157.x
10. Alharthi, H, Alnuaim, H, Aljarbou, O, and Arabi, H. Sebaceous carcinoma of the vulva: A case report and review of the literature. Avicenna J Med (2021) 11(1):49–53. doi:10.4103/ajm.ajm_183_20
11. Schmults, CD, Blitzblau, R, Aasi, SZ, Alam, M, Andersen, JS, Baumann, BC, et al. NCCN Guidelines® insights: Squamous cell skin cancer, version 1.2022. J Natl Compr Canc Netw (2021) 19(12):1382–94. doi:10.6004/jnccn.2021.0059
12. Thakur, BK, Verma, S, Khonglah, Y, and Jitani, A. Multifocal sebaceous carcinoma of the vulva. Indian J Dermatol Venereol Leprol (2017) 83(2):221–4. doi:10.4103/0378-6323.198436
13. Tryggvason, G, Bayon, R, and Pagedar, NA. Epidemiology of sebaceous carcinoma of the head and neck: Implications for lymph node management. Head Neck (2012) 34(12):1765–8. doi:10.1002/hed.22009
14. Van der Zee, AG, Oonk, MH, De Hullu, JA, Ansink, AC, Vergote, I, Verheijen, RH, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol (2008) 26(6):884–9. doi:10.1200/JCO.2007.14.0566
15. Oonk, MHM, Slomovitz, B, Baldwin, PJW, van Doorn, HC, van der Velden, J, de Hullu, JA, et al. Radiotherapy versus inguinofemoral lymphadenectomy as treatment for vulvar cancer patients with micrometastases in the sentinel node: Results of GROINSS-V II. J Clin Oncol (2021) 39(32):3623–32. doi:10.1200/JCO.21.00006
16. Bhaijee, F, and Brown, AS. Muir-Torre syndrome. Arch Pathol Lab Med (2014) 138(12):1685–9. doi:10.5858/arpa.2013-0301-RS
17. Chang, AY, Miller, CJ, Elenitsas, R, Newman, JG, and Sobanko, JF. Management considerations in extraocular sebaceous carcinoma. Dermatol Surg (2016) 42(1):S57–65. doi:10.1097/DSS.0000000000000575
18. Bogani, G, Leone Roberti Maggiore, U, and Raspagliesi, F. Lynch syndrome - muir-torre variant: Implication in gynecologic oncology. J Gynecol Oncol (2018) 29(5):e84. doi:10.3802/jgo.2018.29.e84
Keywords: extraocular sebaceous carcinoma, vulval sebaceous carcinoma, surgery, adjuvant therapy, prognosis
Citation: Wang X and Wei X (2023) Case report: Vulval sebaceous carcinoma: a report of two cases and literature review focus on treatment and survival. Pathol. Oncol. Res. 29:1611259. doi: 10.3389/pore.2023.1611259
Received: 18 April 2023; Accepted: 21 June 2023;
Published: 30 June 2023.
Edited by:
Gabor Cserni, University of Szeged, HungaryCopyright © 2023 Wang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Wei, d2VpeGluMTAxN2FAMTYzLmNvbQ==
 Xiaoxue Wang
Xiaoxue Wang Xin Wei2*
Xin Wei2*