- 1Pathology Department, The First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, China
- 2Breast Surgery, The First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, China
Objective: We aimed to explore the relationship between peripheral blood circulating tumor cells (CTCs) and the expression of Claudin-4 in patients with breast cancer, and further explore the potential impact on clinical prognosis and risk assessment.
Methods: We classified and enumerated circulating tumor cells in the blood of breast cancer patients by CTC-enriched in situ hybridization and the detection of Claudin-4 expression by immunohistochemistry. We carried out an analysis of the correlation between the two and the comparison of their impact on clinical parameters and prognosis.
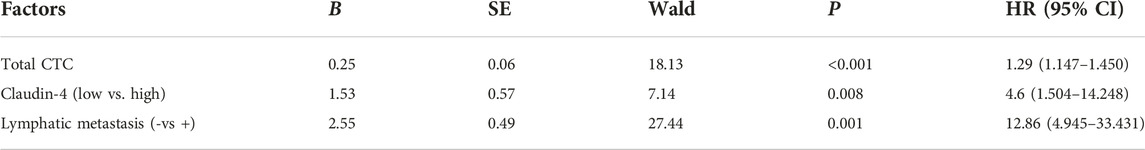
Results: There were 38 patients with a low expression of Claudin-4 and 27 patients with a high expression of Claudin-4. Compared with Claudin-4 low-expression patients, the number of CTCs was higher in patients with high Claudin-4 expression (11.7 vs. 7.4, p < 0.001). High Claudin-4 expression was associated with a lower count of epithelial CTCs (E-CTCs) (3.4 vs. 5.0, p = 0.033), higher counts of mesenchymal CTCs (M-CTC) (4.4 vs. 1.1, p < 0.001), and epithelial/mesenchymal CTCs (E/M-CTCs) (4.0 vs. 3.5, p = 0.021). The intensity of Claudin-4 was positively correlated with CTC (rs = 0.43, p = 0.001). Multivariate COX regression analysis showed that CTC counts (HR = 1.3, p < 0.001), Claudin-4 (HR = 4.6, p = 0.008), and Lymphatic metastasis (HR = 12.9, p = 0.001) were independent factors for poor prognosis. COX regression of CTC classification showed that epithelial/mesenchymal CTCs (E/M-CTC) (HR = 1.9, p = 0.001) and mesenchymal CTCs (M-CTC) (HR = 1.5, p = 0.001) were independent influencing factors of adverse reactions in breast cancer patients.
Conclusion: The number of CTC in breast cancer is positively correlated with the expression of Claudin-4. High CTC counts and a high proportion of M-CTCs correlated with Claudin-4 expression. CTC counts and Claudin-4 expression were independent predictors of poor prognosis in breast cancer patients.
Introduction
Breast cancer is the most common malignant tumor in women [1, 2]. In 2020, there were an estimated 2,261,419 new breast cancer cases, accounting for 11.7% of the total cancer incidence, surpassing lung cancer and becoming the leading cause of cancer death among women [2]. Early (or operable) and non-inflammatory locally advanced inoperable breast cancers are considered potentially curable, but the prognosis is better with lower stages [3–6], emphasizing the need for screening and early detection [7, 8].
Several countries have breast cancer screening programs based on mammography, and these programs are associated with a 15%–30% decrease in breast cancer-related mortality but not in overall mortality [8–10]. Breast cancer screening programs are mainly based on mammography, and their sensitivity and specificity remain relatively low, leading to several false-positive and false-negative results [8–11]. Therefore, it is particularly important to find highly sensitive indicators of prognosis and recurrent risks of breast cancer [12].
A small amount of circulating tumor cells (CTCs) in peripheral blood can escape the tumor after the epithelial-mesenchymal transition [13]. In addition, monitoring peripheral blood CTCs could fill the diagnostic gap in tumors <5 mm [14]. The monitoring of CTCs is convenient and conducive to the early diagnosis and prognosis evaluation of breast cancer [15, 16]. They act as seeds for metastases and can be classified as epithelial type (E-CTC), mesenchymal type (M-CTC), or intermediate (E/M-CTC) with a transition from epithelial to mesenchymal phenotype. These subtypes can be distinguished based on the expression of surface markers. Claudin-4 is a tight junction protein family member and mainly regulates tight junctions [17]. Therefore, Claudin-4 plays an important role in the occurrence and metastasis of cancer cells [18]. Studies have shown that Claudin-4 is highly expressed in various malignant tumors [19], suggesting that Claudin-4 may become a tumor marker and an emerging target for treatment [20–22]. Claudin-4 has been reported to regulate EMT through p21-activated kinase 4 (PAK4) expression in human breast cancer cells [23]. However, the relationship between CTCs and Claudin-4 in breast cancer is unclear.
Therefore, we speculate that Claudin-4 may be involved in the EMT process by altering CTC numbers and typing. This study aimed to explore the relationship between peripheral blood CTCs and the expression of Claudin-4 in patients with breast cancer and their relationship with the survival and prognosis of breast cancer patients.
Methods
Study design and patients
This cross-sectional study included patients who underwent routine surgical resection and were pathologically confirmed as having breast cancer at the First Affiliated Hospital of Henan University of Traditional Chinese Medicine between January 2018 and December 2020. This study was approved by the ethics committee of the First Affiliated Hospital of Henan University of Traditional Chinese Medicine. The requirement for informed consent was waived due to the retrospective nature of the study. The inclusion criteria were: 1) available clinical data, 2) diagnosis of breast cancer and available tissue specimens, and 3) available peripheral blood samples. The exclusion criteria were: 1) incomplete clinical data or 2) recipient of neoadjuvant treatments.
Data collection
The demographic and clinical data of the patients were collected, including age, sex, lymph node metastasis, carcinogenic molecular detection, pathological type, tumor size, and Ki-67 expression. Molecular subtypes included Luminal A, Luminal B, human epidermal growth factor receptor-2 (HER-2) amplification, and basal-like. Luminal A type showed estrogen-receptor (ER) positive or progesterone-receptor (PR) positive and PR high expression (≥20%), HER2 negative, or Ki-67 low expression (<14%). Luminal (luminal or hormone receptor-positive) type B was divided into two subtypes, Luminal B (HER-2 negative) and Luminal B (HER-2 positive): Luminal B (HER-2 negative): ER and/or PR positive, HER-2 negative, and high expression of Ki-67 (greater than or equal to 14%). Luminal B (HER-2 positive): ER and/or PR positive, HER- 2 overexpression or proliferation, and any level of Ki-67. HER-2 amplification type, also known as HER2 positive (non-Luminal) type, showed ER and PR deletion, HER-2 overexpression or proliferation, and any level of Ki-67. Basal-like breast cancers have low expressions of ER, PR, and HER2 and high expressions of markers of breast basal or myoepithelial cells, such as caveolin-1, p63.
CTC detection
Peripheral venous blood (5 mL) was collected from an antecubital vein using a vacuum EDTA anticoagulant tube and transferred to a sample storage tube. The tube was inverted >10 times and incubated at 15°C–30°C for 30 min to lyse the cells. The blood was centrifuged at 1850 rpm for 5 min. The supernatant was discarded, and 4 mL of PBS and 1 mL of RI fixative solution were added, vortexed, and let to stand for 8 min at room temperature. The liquid was poured into the filter connected to a vacuum pump with a negative pressure of −0.06 MPa. The fixative (1 mL) was added for 1 h. The membrane was dehydrated with gradient alcohol to capture the cells. Labels for EP-CAM, CK8, and CK8/18 (as epithelial markers) and Vimentin (as mesenchymal markers) produced by CanPatrol Biotechnology Co., Ltd. were used for mRNA fluorescence in situ hybridization. Fluorescence from any of the epithelial/mesenchymal makers are indicative of epithelial/mesenchymal-type circulating tumor cells. The membrane was scanned, and the count of each type of cell was analyzed using Isis5 and Metafer3 fluorescence analysis software produced by German Midas. Red fluorescence represented epithelial-type CTCs (E-CTC), green fluorescence represented mesenchymal-type CTCs (M-CTC), and both red and green represented mixed-type CTCs (E/M-CTC).
Immunohistochemistry
The postoperative specimens were fixed in 10% formalin, dehydrated, paraffin-embedded, and serially cut into 4-μm sections. After dewaxing and rehydrating, the sections were put into ethylenediaminetetraacetic acid (EDTA) solution (pH 8.0) produced by Beijing Soleibao Technology Co., Ltd. at 100°C for 20 min. The endogenous peroxidase activity was blocked with 3% H2O2 for 10 min. After rinsing with phosphate buffer solution (PBS), the sections were incubated with an anti-rabbit primary antibody for Claudin-4 (Shanghai Jiehao Biotechnology Technology Co., Ltd., CRM-1251) at 37°C for 1 h. After washing with PBS, the sections were incubated with the secondary antibody (Roche Shanghai Co., Ltd., J19565) and rinsed. The sections were revealed with diaminobenzidine (DAB), stained with hematoxylin, dehydrated, and mounted. The cells were counted in 10 randomly selected visual fields at ×400 magnification. For number scoring, <5% of positive cells were scored zero, 5%–25% was one, 26%–50% was two, and >50% was three. For intensity scoring, no staining was zero, light yellow was one, brown was two, and tan was three. The number and intensity scores were multiplied; zero points were negative, one to two represented 1+, three to four represented 2+, and five to six represented 3+. Claudin-4 negative and Claudin-4 1+ were considered low expressions. Claudin-4 2+ and 3+ were considered high expressions. The scores were determined by two pathologists with 10 years of experience, and the average value was taken as the final result.
Follow-up
The patients were followed up every 2 months by telephone contact after enrollment. Patients’ vital signs and disease progression were recorded. If necessary, the patients were admitted to the hospital to evaluate their progress. The expected follow-up time was 24 months. The follow-up endpoint was all-cause death. The follow-up deadline was 30 October 2020.
Statistical analysis
All data were analyzed using SPSS 20.0 (IBM, Armonk, NY, United States). Continuous data were expressed as means ± standard deviation and analyzed using Student’s t-test. Categorical data were presented as n (%) and analyzed using the chi-square test. The relationship between CTCs and Claudin-4 expression was analyzed using Spearman rank correlation and the Kaplan-Meier method was used to draw the survival curve. Log-rank test was used to compare the differences. A Multivariate COX risk regression model was established to analyze the independent adverse factors of prognosis. The statistical analysis of the data from two groups was performed using a t-test. The comparisons of multiple groups were performed by one-way ANOVA and then an LSD-t test. p < 0.05 was considered to be significant. Two-sided p-values < 0.05 were considered statistically significant.
Results
Characteristics of the patients
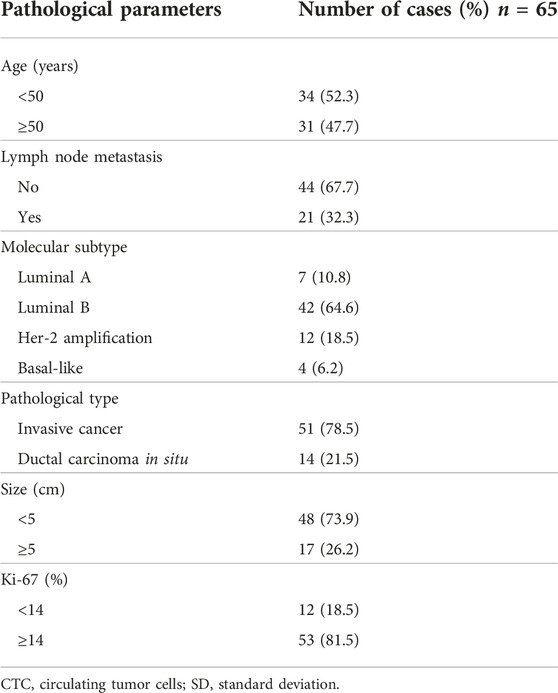
Sixty-five breast cancer patients, 39 to 61 (median, 49) years of age, were included. All patients were women, including 51 with invasive carcinoma and 14 cases of ductal carcinoma in situ. There were 48 patients with a tumor <5 cm and 17 with a tumor >5 cm. There were 21 patients with lymph node metastasis and 44 without. There were 12 patients with Ki- 67 < 14% and 53 with Ki-67 ≥ 14% (Table 1).
Claudin-4
Among the 65 patients, there were 11 Claudin-4-negative tumors, 27 with Claudin-4 +, 22 with Claudin-4 2+, and five with Claudin-4 3+ (Figure 1). Most patients with high Claudin-4 expression had lymph node metastasis (55.6% vs. 15.8%, p < 0.001 vs. without low Claudin-4 expression). Most patients with high Claudin-4 expression had a tumor >5 cm (51.9% vs. 7.9%, p < 0.001 vs. low Claudin-4 expression) (Table 2).
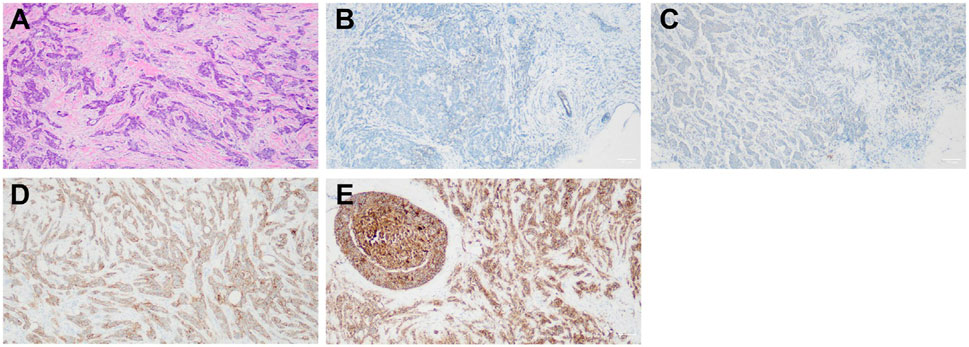
FIGURE 1. (A) Non-specific invasive carcinoma with HE staining (×100). (B) Non-specific invasive carcinoma with negative claudin-4 (SP ×100). (C) Non-specific invasive carcinoma claudin-4 (+, SP ×100). (D) Non-specific invasive carcinoma claudin-4 (2+, SP ×100). (E) Non-specific invasive carcinoma claudin-4 (3+, SP ×100).
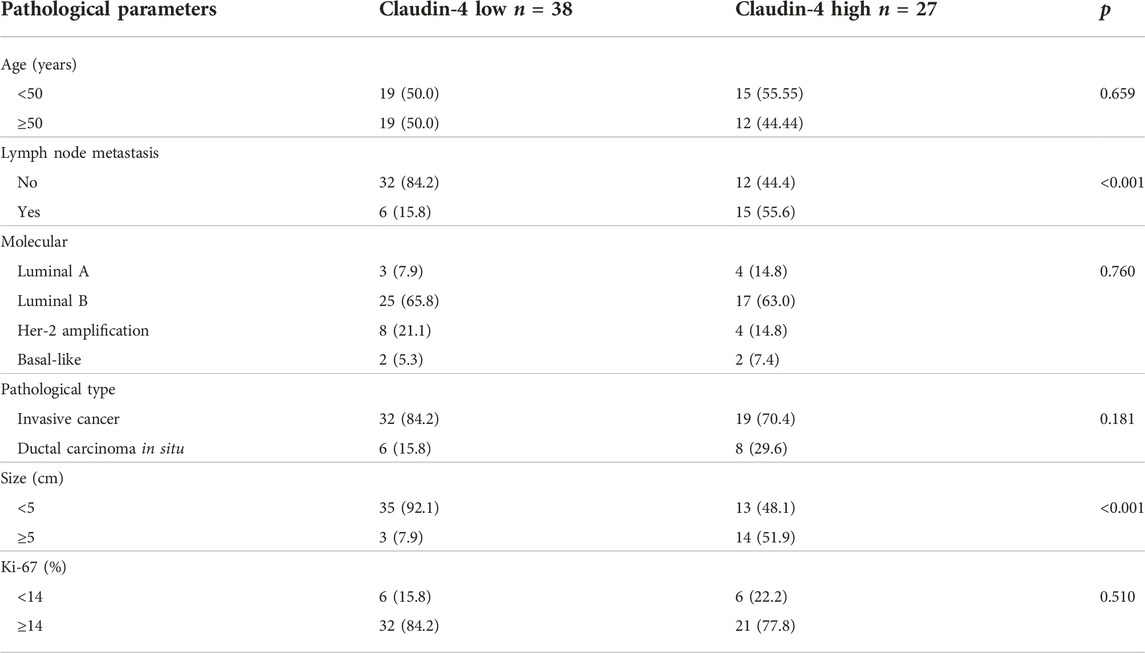
TABLE 2. Comparison of Claudin-4 in patients’ age, lymph node metastasis, and molecular classification.
Circulating tumor cells
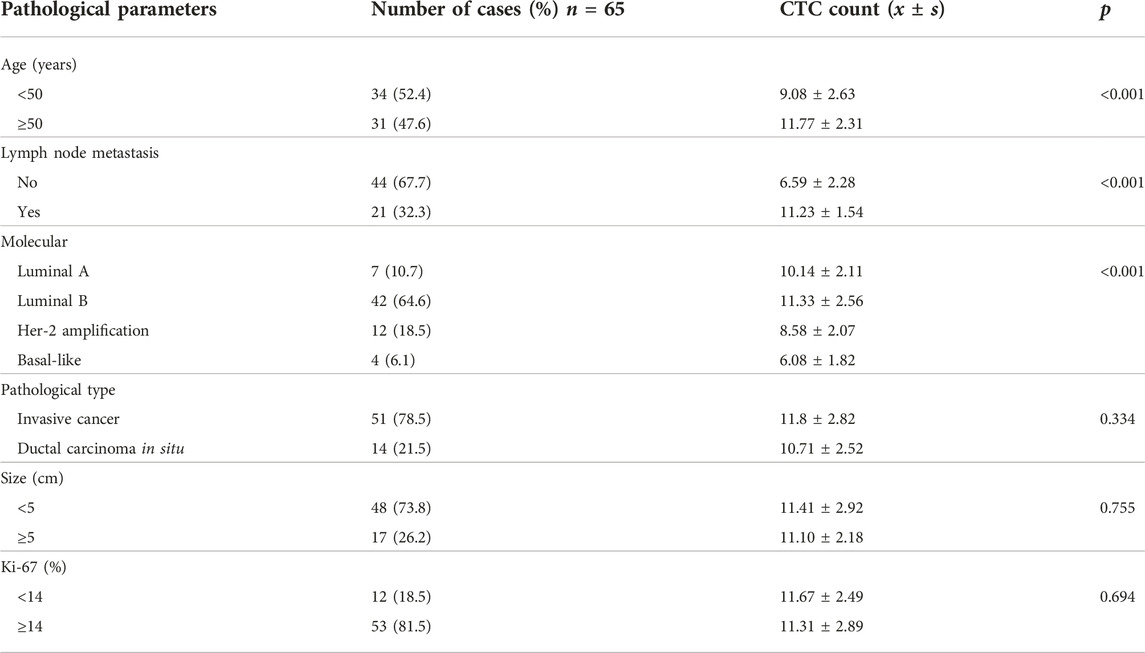
CanPatrol CTC enrichment and in situ hybridization were used to monitor the number and classification of CTC in the blood of 65 patients. Red fluorescence was used to represent epithelial CTC, and green fluorescence was used to represent interstitial CTC. CTC was divided into epithelial type (E-CTC), mixed type (E/M-CTC), and interstitial type (M-CTC). The number of CTCs in patients aged ≥50 was significantly high (11.8 vs. 9.1, p < 0.001 vs. Age < 50). The number of CTCs in patients with lymph node metastasis was high (11.2 vs. 6.5, p < 0.001 vs. without lymph node metastasis). There are significant differences in the number of CTCs in different molecular types of breast cancer (p < 0.001) (Table 3).
Circulating tumor cells and Claudin-4
The number of CTCs was higher in patients with high Claudin-4 expression (11.7 vs. 7.4, p < 0.001). A more detailed analysis revealed that a high Claudin-4 expression was associated with a lower count of E-CTCs (3.4 vs. 5.0, p = 0.033) but high counts of E/M-CTCs (5.0 vs. 3.5, p = 0.021) and M-CTCs (4.4 vs. 1.1, p < 0.001) (Table 4 and Figure 2). Spearman correlation analysis showed that the intensity of Claudin-4 was positively correlated with CTC (rs = 0.43, p = 0.001) (Figure 3).
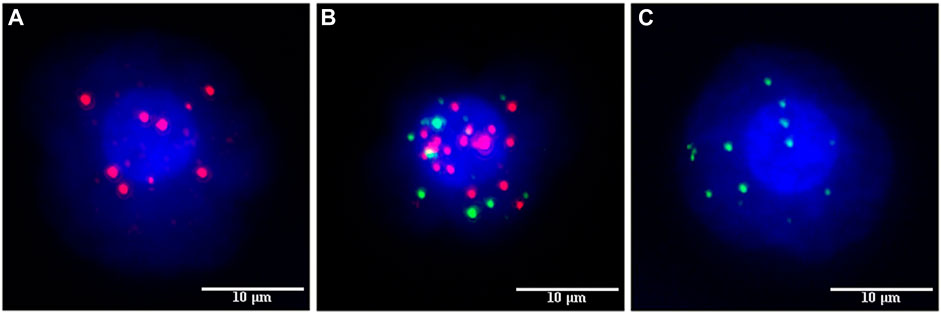
FIGURE 2. Fluorescence marking different types of circulating tumor cells (CTCs). (A) EPCAM, CK8, and cK8/18 epithelial fluorescent markers emit red fluorescence to label epithelial circulating tumor cells. (B) Hybrid circulating tumor cells were labeled with both red and green fluorescence. (C) Vimentin mesenchymal fluorescent marker emits green fluorescence to label mesenchymal circulating tumor cells.
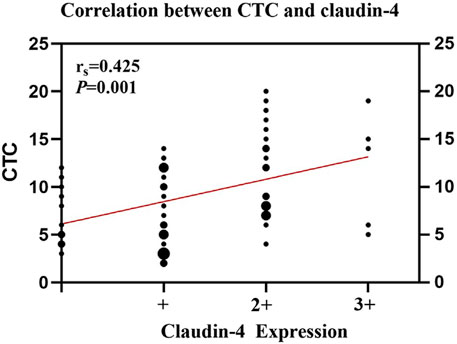
FIGURE 3. Spearman rank correlation analysis between circulating tumor cells (CTCs) and Claudin-4 expression.
Correlation analysis between CTC classification and lymph node metastasis
E-CTC in breast cancer patients with lymph node metastasis was significantly higher than that in the lymph node metastasis group (3.4 vs. 1.4, p < 0.001). E/M-CTC was significantly lower than that in the lymph node metastasis group (1.5 vs. 4.3, p = 0.002), and the M-CTC count was lower than that in the lymph node metastasis group (1.6 vs. 6.4, p < 0.001) (Table 5).
Survival analysis of Claudin-4 expression level cancer
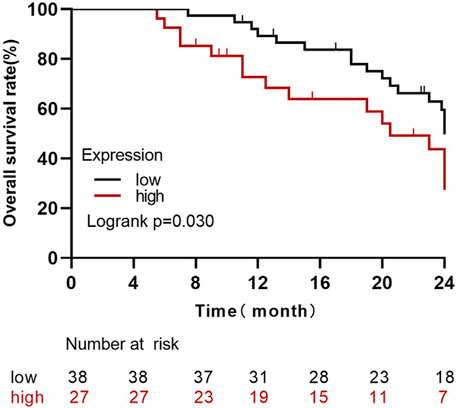
By the last follow-up, the average survival time of 65 patients with high expressions of Claudin-4 (n = 27) was 18.22 months, and the average survival time of the low-expression group (n = 38) was 21.81 months. The survival rate of the high-expression group was significantly lower than that of the low-expression group (Log Rank Χ2 = 4.71, p = 0.030) (Figure 4).
A multivariate COX regression analysis model was constructed
The results showed that CTC counts (HR = 1.3, p < 0.001), Claudin-4 (HR = 4.6, p = 0.008), and lymph node metastasis (HR = 12.9, p = 0.001) were independent prognostic factors for poor prognosis (Table 6).
The COX risk regression model of CTC classification and prognosis of breast cancer patients was constructed
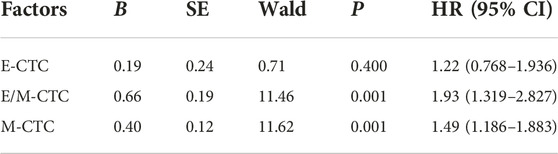
Our analysis showed that E/M-CTC (HR = 1.9, p = 0.001) and M-CTC (HR = 1.5, p = 0.001) were independent influencing factors of poor prognosis in breast cancer patients (Table 7).
Discussion
Breast cancer with metastases can reduce survival rates, so markers for early diagnosis and prognostic monitoring are urgently needed for breast cancer patients. Based on this study, we can explore the prognosis of patients by assessing the expression of Claudin-4 and circulating tumor cells in peripheral blood.
The results showed that the CTC count and classification in breast cancer are associated with the expression of Claudin-4. The results may help define novel molecular targets for the early diagnosis and prognosis of breast cancer. Future studies should examine the diagnostic and prognostic values of CTCs and Claudin-4 in breast cancer patients.
Claudins are the most important structural and functional component of tight junction transmembrane proteins and can maintain cell-to-cell molecular flow and cell polarity [24]. Claudins are overexpressed or silenced in pancreatic cancer, colon cancer, ovarian cancer, breast cancer, and other solid tumors [25]. In particular, Claudin-4 can directly or indirectly promote tumor metastasis through the second extracellular loop structure [26]. The changes in the expression of Claudin-4 can modify the structure of the tight junctions and adhesion between cells, leading to tumor metastasis and spread [27]. Claudin-4 is overexpressed in ovarian and breast cancers [28]. Kolokytha et al. [29] showed that the positive expression of Claudin-4 in triple-negative breast cancer might be a marker of good prognosis. Radi et al. [30] showed that Claudin-4 was related to the expression of D240 (a lymphatic vessel marker) in prostate cancer, and Claudin-4 is associated with lymph node metastasis and a marker of poor prognosis. Moreover, the present study also confirmed that the expression level of Claudin-4 was associated with lymph node metastasis and tumors ≥5 cm, suggesting that higher Claudin-4 expression may be associated with a poor prognosis in breast cancer. In our study, Claudin-4 was poorly significantly associated with molecular subtypes of breast cancer, and Sara Ricardo’s study showed that a single IHC for Claudin-4 is not sufficient to assess and identify molecular subtypes of breast cancer and that additional markers, such as CSC (cancer stem cell) markers, are needed to improve subgroup identification [31]. The survival time of the Claudin-4 high-expression group was significantly lower than that of the low-expression group, and a multivariate COX regression model was established to find that high expression of Claudin-4 was an independent factor affecting the poor prognosis of breast cancer.
The present study showed that the number of E/M-CTC and M-CTC in the lymph node metastasis group was higher than that in the lymph node non-metastasis group and the numbers of CTCs in the high Claudin-4 group were higher than in the low expression level group, mainly due to the E/M- and M-CTCs since the E-CTCs were lower in the high Claudin-4 group. These results suggest that Claudin-4 is involved in EMT and tumor metastasis and recurrence. The Spearman correlation analysis also showed that CTCs in peripheral blood were positively correlated with the expression level of Claudin-4. The Multivariate COX regression model showed that Claudin-4, lymph node metastasis, and CTC classification E/M-CTC and M-CTC were adverse factors for the prognosis of breast cancer patients. Therefore, both Claudin-4 and CTC might be used to evaluate the prognosis and recurrence risk of patients with breast cancer. Meanwhile, the high expression of Claudin-4 might regulate or be regulated by the tumor EMT process, but the present study could not determine cause-to-effect relationships.
This study has limitations. It was a single-center study with a small sample size. Only CTCs and Claudin-4 were examined. Future studies should look at the correlations of multiple biomarkers and in-depth mechanism research to help personalize the prognosis of breast cancer. It was a cross-sectional study, and cause-to-effect relationships could not be determined.
In conclusion, CTC count and classification in breast cancer are associated with the expression of Claudin-4. CTC count and classification and the expression level of Claudin-4 may be used for the early diagnosis and prognosis of breast cancer. Future large studies should attempt to investigate the diagnostic and prognostic value of CTCs and claudin-4 in breast cancer patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the ethics committee of the First Affiliated Hospital of Henan University of Traditional Chinese Medicine. The requirement for informed consent was waived due to the retrospective nature of the study.
Author contributions
Material preparation, data collection, and analysis were performed by JC, XL, and XH. The first draft of the manuscript was written by CW. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the help of the breast surgery department of our hospital.
References
1. Siegel, RL, Miller, KD, Fuchs, HE, and Jemal, A. Cancer statistics, 2022. Ca: A Cancer J Clin (2022) 72(1):7–33. doi:10.3322/caac.21708
2. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca: A Cancer J Clin (2021) 71(3):209–49. doi:10.3322/caac.21660
3. Harbeck, N, and Gnant, M. Breast cancer. Lancet (2017) 389(10074):1134–50. doi:10.1016/s0140-6736(16)31891-8
4. Senkus, E, Kyriakides, S, Ohno, S, Penault-Llorca, F, Poortmans, P, Rutgers, E, et al. Primary breast cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2015) 26(5):v8–30. doi:10.1093/annonc/mdv298
5. Cardoso, F, Costa, A, Senkus, E, Aapro, M, André, F, Barrios, CH, et al. 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Ann Oncol (2017) 28(1):16–33. doi:10.1093/annonc/mdw544
6.Nccn Clinical Practice Guidelines in Oncology (Nccn Guidelines). Breast cancer. Version 2.2022. Fort Washington: National Comprehensive Cancer Network (2021).
7.Nccn Clinical Practice Guidelines in Oncology (Nccn Guidelines). Breast cancer risk reduction. Version 1.2022. Fort Washington: National Comprehensive Cancer Network (2022).
8.Nccn Clinical Practice Guidelines in Oncology (Nccn Guidelines). Breast cancer screening and diagnosis. Version 1.2021. Fort Washington: National Comprehensive Cancer Network (2021).
9. Gøtzsche, PC, and Jørgensen, KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev (2013) 2013(6):Cd001877. doi:10.1002/14651858
10. Myers, ER, Moorman, P, Gierisch, JM, Havrilesky, LJ, Grimm, LJ, Ghate, S, et al. Benefits and harms of breast cancer screening: A systematic review. Jama (2015) 314(15):1615–34. doi:10.1001/jama.2015.13183
11. Nelson, HD, Fu, R, Cantor, A, Pappas, M, Daeges, M, and Humphrey, L. Effectiveness of breast cancer screening: Systematic review and meta-analysis to update the 2009 U.S. Preventive services task force recommendation. Ann Intern Med (2016) 164(4):244–55. doi:10.7326/m15-0969
12. Liu, X, Tang, JH, and Wang, MS. Expression of claudin-4 in human breast cancer tissue and clinical significance. J Jainan Med Coll (2014) 20(12):1607–13. doi:10.13210/j.cnki.jhmu.20140926.005
13. Li, R, Gong, Z, Liu, Y, Zhao, X, and Guo, SJNF. Detection of circulating tumor cells and single cell extraction Technology: Principle, effect and application prospect. Futures (2021) 5(3):032002. doi:10.1088/2399-1984/ac1325
14. Wu, F, Zhao, J, and Zhang, X. Comparison of value of circulating tumor cells and tumor markers in lung cancer diagnosis. Int J Inspection Med (2020) 41(5):527–31. doi:10.3969/j.issn.1673-4130.2020.05.004
15. Thery, L, Meddis, A, Cabel, L, Proudhon, C, Latouche, A, Pierga, JY, et al. Circulating tumor cells in early breast cancer. JNCI Cancer Spectr (2019) 3(2):pkz026. doi:10.1093/jncics/pkz026
16. Zhang, H, Lin, X, Huang, Y, Wang, M, Cen, C, Tang, S, et al. Detection methods and clinical applications of circulating tumor cells in breast cancer. Front Oncol (2021) 11:652253. doi:10.3389/fonc.2021.652253
17. Luo, L, Ma, GR, and Liu, FG. Expression and clinical significance of tight junction proteins 1,3 and 4 in breast cancer patients. J Difficult Dis (2017) 16(06):610. doi:10.3969/j.issn.1671-6450.2017.06.017
18. Lee, KW, Lee, NK, Kim, JH, Kang, MS, Yoo, HY, Kim, HH, et al. Twist1 causes the transcriptional repression of claudin-4 with prognostic significance in esophageal cancer. Biochem Biophys Res Commun (2012) 423(3):454–60. doi:10.1016/j.bbrc.2012.05.140
19. Xiang, RL, Su, YC, and Pei, XQ. Progress in functions of tight junction protein claudin-4. Sheng Li Ke Xue Jin Zhan [Progress Physiol] (2012) 43(4):310–4. doi:10.3969/j.issn.0559-7765
20. Abd-Elazeem, MA, and Abd-Elazeem, MA. Claudin 4 expression in triple-negative breast cancer: Correlation with androgen receptors and ki-67 expression. Ann Diagn Pathol (2015) 19(1):37–42. doi:10.1016/j.anndiagpath.2014.10.003
21. Luo, Y, Kishi, S, Sasaki, T, Ohmori, H, Fujiwara-Tani, R, Mori, S, et al. Targeting claudin-4 enhances chemosensitivity in breast cancer. Cancer Sci (2020) 111(5):1840–50. doi:10.1111/cas.14361
22. Duarte, GM, Almeida, NR, Tocchet, F, Espinola, J, Barreto, CTR, Pinto, GA, et al. Claudin-4 expression is associated with disease-free survival in breast carcinoma-in-situ: Mean follow-up of 8.2 years. Clin Breast Cancer (2018) 18(5):e1111–6. doi:10.1016/j.clbc.2018.06.005
23. Wang, F, Gao, Y, Tang, L, Ning, K, Geng, N, Zhang, H, et al. A novel pak4-cebpb- cldn4 Axis involving in breast cancer cell migration and invasion. Biochem Biophys Res Commun (2019) 511(2):404–8. doi:10.1016/j.bbrc.2019.02.070
24. Singh, AB, and Dhawan, P. Claudins and cancer: Fall of the soldiers entrusted to protect the gate and keep the barrier intact. Semin Cel Dev Biol (2015) 42:58–65. doi:10.1016/j.semcdb.2015.05.001
25. Tabariès, S, and Siegel, PM. The role of claudins in cancer metastasis. Oncogene (2017) 36:1176–90. doi:10.1038/onc.2016.289
26. Webb, PG, Spillman, MA, and Baumgartner, HK. Claudins play a role in normal and tumor cell motility. BMC Cel Biol (2013) 14:19. doi:10.1186/1471-2121-14-19
27. Escudero-Esparza, A, Jiang, WG, and Martin, TA. The claudin family and its role in cancer and metastasis. Front Biosci (Landmark Edition) (2011) 16(3):1069–83. doi:10.2741/3736
28. Fabisiewicz, A, Szostakowska-Rodzos, M, Zaczek, AJ, and Grzybowska, EA. Circulating tumor cells in early and advanced breast cancer; biology and prognostic value. Int J Mol Sci (2020) 21(5):1671. doi:10.3390/ijms21051671
29. Kolokytha, P, Yiannou, P, Keramopoulos, D, Kolokythas, A, Nonni, A, Patsouris, E, et al. Claudin-3 and claudin-4: Distinct prognostic significance in triple-negative and luminal breast cancer. Appl Immunohistochem Mol Morphol (2014) 22(2):125–31. doi:10.1097/PAI.0b013e31828d9d62
30. Radi, DA, and Abd-Elazeem, MA. Prognostic significance of lymphatic vessel density detected by D2-40 and its relation to claudin-4 expression in prostatic adenocarcinoma. Int J Surg Pathol (2016) 24(3):219–26. doi:10.1177/1066896915611488
Keywords: breast cancer, circulating tumor cells, tight junction protein, claudin-4, molecular subtype
Citation: Chai J, Liu X, Hu X and Wang C (2023) Correlation analysis of circulating tumor cells and Claudin-4 in breast cancer. Pathol. Oncol. Res. 29:1611224. doi: 10.3389/pore.2023.1611224
Received: 04 April 2023; Accepted: 14 June 2023;
Published: 03 July 2023.
Edited by:
Anna Sebestyén, Semmelweis University, HungaryCopyright © 2023 Chai, Liu, Hu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunfang Wang, YXNhbmduYTAwOUAxNjMuY29t
 Jie Chai1
Jie Chai1 Chunfang Wang
Chunfang Wang