- Department of Thoracic Surgery, People’s Hospital of Deyang City, Deyang, China
Background and purpose: The association between the pretreatment Controlling Nutritional Status (CONUT) score and the prognosis of esophageal cancer patients remains unclear. The aim of this meta-analysis was to further elucidate the prognostic role of the pretreatment CONUT score in esophageal cancer based on current evidence.
Methods: The PubMed, Embase, Web of Science and CNKI databases were searched up to 27 September 2022. The primary and secondary outcomes were overall survival (OS) and progression-free survival (PFS)/cancer-specific survival (CSS), and the hazard ratio (HR) and 95% confidence interval (CI) were pooled for analysis.
Results: A total of 11 retrospective studies involving 3,783 participants were included. The pooled results demonstrated that a higher pretreatment CONUT score was significantly related to poor OS (HR = 1.82, 95% CI: 1.31–2.54, p < 0.001), and subgroup analysis stratified by pathological type showed similar results. In addition, the pretreatment CONUT score was associated with poor PFS (HR = 1.19, 95% CI: 1.10–1.28, p < 0.001) and CSS (HR = 2.67, 95% CI: 1.77–4.02, p < 0.001).
Conclusion: The pretreatment CONUT score was predictive of worse prognosis in esophageal cancer, and patients with a higher CONUT score showed worse survival.
Introduction
Esophageal carcinoma is one of the most common malignant cancers worldwide, ranking sixth and seventh in terms of mortality and incidence [1, 2]. To date, the prognosis of patients with esophageal cancer remains extremely poor despite considerable advances in surgical technologies, neoadjuvant chemotherapy and immunotherapy [3]. The TNM stage is regarded as the prognostic indicator for esophageal cancer and contributes substantially to the formulation of a treatment strategy. However, increasing evidence indicates that a number of host parameters affect the long-term survival of esophageal cancer [4].
In particular, nutritional status has been verified as an essential prognostic factor in cancers, and it has been reported that impaired nutritional conditions have a negative impact on survival in cancer patients [5]. Thus, a number of nutritional indicators based on laboratory data have been investigated, and the prognostic role of several indices has been explored in recent years, including the prognostic nutritional index (PNI) [6], nutritional risk index (NRI) [7], geriatric nutritional risk index (GNRI) [8] and modified Glasgow Prognostic Score (mGPS) [9]. Some of them have been shown to be significantly associated with the prognosis of esophageal carcinoma patients [6, 8, 9]. The Controlling Nutritional Status (CONUT) score is a novel nutritional indicator that has been described in the past several years [10]. It is calculated by scoring serum, albumin concentration, lymphocyte count and total cholesterol level [11], and the association of CONUT score and perioperative surgical risk in esophageal cancer has been reported [12]. In addition, the relationship between the pretreatment CONUT score and survival has also been manifested in several types of tumors by meta-analyses, including renal cell carcinoma, pancreatic cancer, lung cancer and colorectal cancer [13–16]. However, whether the pretreatment CONUT score could serve as a reliable prognostic factor in esophageal cancer is unclear.
Therefore, this meta-analysis aimed to further elucidate the association of the pretreatment CONUT score with long-term survival in esophageal cancer, which might contribute to the treatment strategy for esophageal cancer patients.
Materials and methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analyses 2020 [17].
Literature search
The PubMed, Embase, Web of Science and CNKI databases were searched up to 27 September 2022, for studies investigating the prognostic role of the pretreatment CONUT score in esophageal cancer. The following terms were used during the search: Controlling Nutritional Status score, CONUT, esophageal, esophagus, cancer, tumor, carcinoma, neoplasm, survival, prognostic and prognosis. The specific search strategy was as follows: (Controlling Nutritional Status score OR CONUT) AND (esophageal OR esophagus) AND (cancer OR tumor OR carcinoma OR neoplasm) AND (survival OR prognostic OR prognosis). Additionally, all references cited in the included studies were also screened.
Inclusion criteria
The inclusion criteria were as follows: 1) patients were diagnosed with primary esophageal cancer pathologically; 2) the CONUT score was calculated based on the serum albumin concentration, peripheral total lymphocyte count and cholesterol level before antitumor treatment as previously described: serum albumin levels≥3.50 g/dL, 3.00–3.49 g/dL, 2.50–3.49 g/dL and <2.50 g/dL [18] were separately defined as score 0, 2, 4 and 6, the total cholesterol levels≥180 mg/dL, 140–179 mg/dL, 100–139 mg/dL and<100 mg/dL were scored 0, 1, 2 and 3 and the total lymphocyte counts ≥1,600/µL, 1,200–1,599/µL, 800–1,199/µL and<800/µL were scored 0, 1, 2 and 3, respectively; 3) patients were divided into two groups based on the CONUT score and the association between pretreatment CONUT score and prognosis presenting as the overall survival (OS), progression-free survival (PFS) or cancer-specific survival (CSS); 4) the hazard ratios (HRs) and 95% confidence intervals (CIs) for the above endpoints were provided in the papers or the Kaplan‒Meier survival curves were presented; and 5) high-quality studies with a Newcastle‒Ottawa Scale (NOS) score >5 [19].
Exclusion criteria
The exclusion criteria were as follows: 1) publications with letters, editorials, case reports, reviews or animal trials; 2) overlapped or duplicated data; and 3) the HRs with corresponding 95% CIs were not available.
Data collection
The following information was extracted: the name of the first author, publication year, sample size, country, tumor-node-metastasis (TNM) stage, treatment (surgical or nonsurgical therapy), pathological type, definition and comparison of CONUT score, endpoint, HR and 95% CI.
Methodological quality assessment
The quality was assessed according to the NOS score tool, and only high-quality studies with an NOS score ≥5 were ultimately included [19].
The literature search, selection, data collection and quality assessment were all conducted by two authors independently, and any disagreement was resolved by discussion.
Statistical analysis
All statistical analyses were performed using STATA 15.0 software. The HRs and 95% CIs were calculated to evaluate the association between the pretreatment CONUT score and the prognosis of esophageal cancer patients. Heterogeneity among the included studies was evaluated using I2 statistics and the Q-test. If obvious heterogeneity was observed, representing I2 > 50% and/or p < 0.1, the random effects model was applied; otherwise, the fixed effects model was used. Sensitivity analysis was performed to clarify sources of heterogeneity and evaluate the stability of the pooled results. Additionally, Begg’s test and Egger’s test were conducted to detect publication bias, and significant publication bias was defined as p < 0.05 [20, 21]. If we detected significant publication bias, then the nonparametric trim-and-fill method was used to re-estimate a corrective effect size after publication bias was adjusted [22].
Results
Literature search and selection
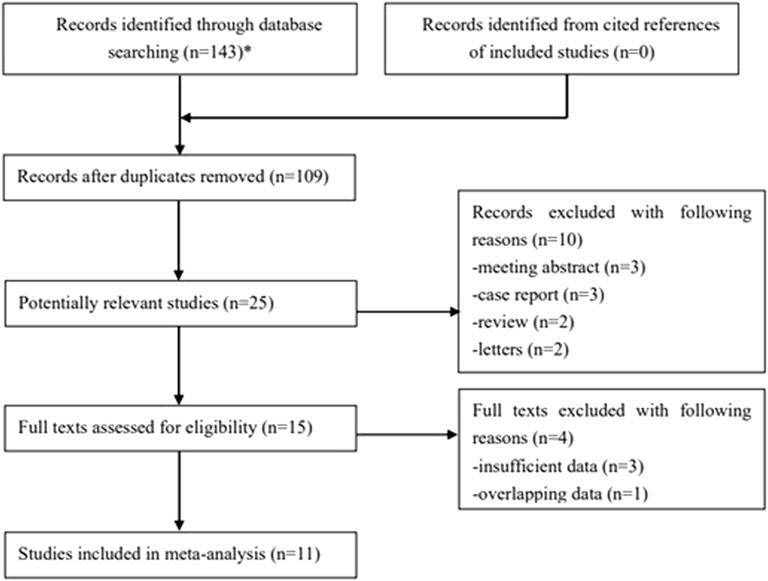
One hundred forty-three records were searched from databases, and 34 duplicated publications were removed. Then, 25 potentially relevant studies were further reviewed after excluding 84 irrelevant publications by reading the titles. The full texts of the remaining 15 studies were carefully reviewed after excluding 10 publications. Ultimately, 11 retrospective studies were included [23–33]. The specific process is displayed in Figure 1.
Basic characteristics of the included studies
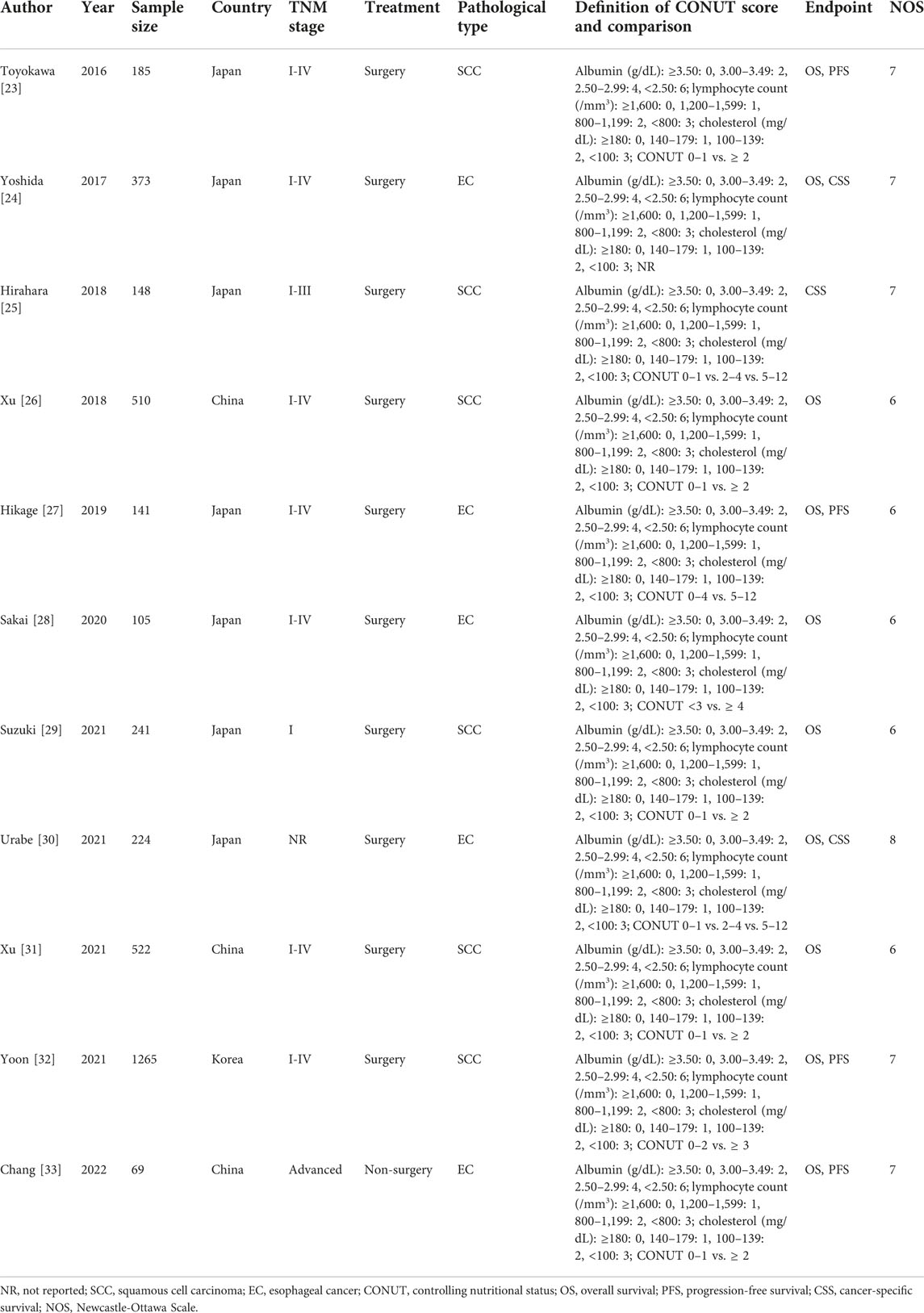
A total of 3,783 patients were enrolled, and most (7/11) studies were from Japan [23–25, 27–30]. Except for the study by Chang et al. [33], the other studies included operated patients. In addition, six studies focused on esophageal squamous cell carcinoma (ESCC) patients [23, 25, 26, 29, 31, 32]. The other detailed characteristics are presented in Table 1.
Predictive effect of the pretreatment CONUT score for OS
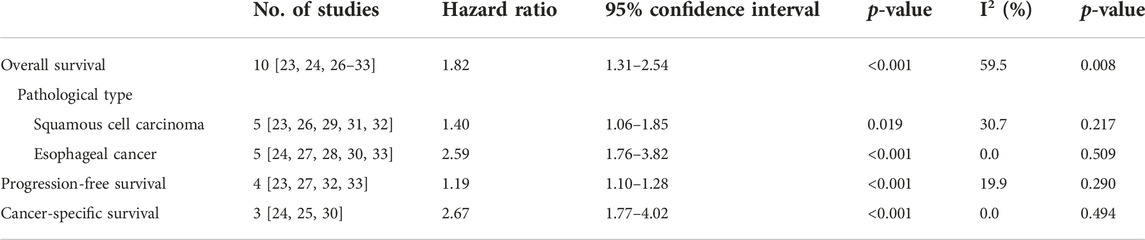
Ten studies explored the predictive effect of the pretreatment CONUT score on OS in esophageal cancer [23, 24, 26–33]. The results demonstrated that a higher pretreatment CONUT score was obviously related to poorer OS (HR = 1.82, 95% CI: 1.31–2.54, p < 0.001; I2 = 59.5%, p = 0.008) (Figure 2). Subgroup analysis based on the pathological type showed similar results (ESCC: HR = 1.40, 95% CI: 1.06–1.85, p = 0.019; esophageal cancer: HR = 2.59, 95% CI: 1.76–3.82, p < 0.001) (Table 2).
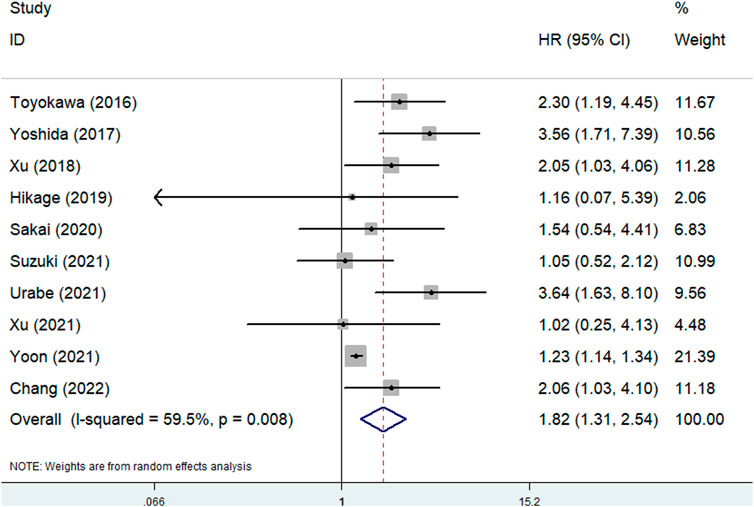
FIGURE 2. The association between pretreatment CONUT score and overall survival of esophageal cancer patients.
Predictive effect of pretreatment CONUT score on PFS and CSS
Four [23, 27, 32, 33] and three [24, 25, 30] studies explored the association between pretreatment CONUT score and PFS and CSS, respectively. The pooled results indicated that a higher pretreatment CONUT score predicted worse PFS (HR = 1.19, 95% CI: 1.10–1.28, p < 0.001; I2 = 19.9%, p = 0.290) and CSS (HR = 2.67, 95% CI: 1.77–4.02, p < 0.001; I2 = 0.0%, p = 0.494) (Figures 3A, B; Table 2).
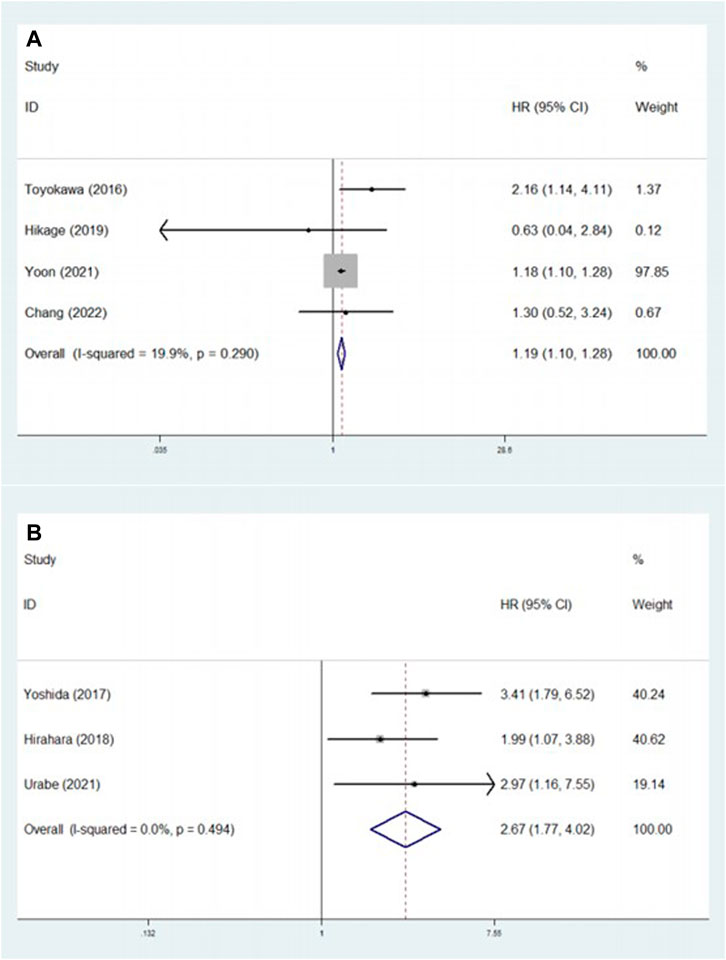
FIGURE 3. (A) The association between pretreatment CONUT score and progression-free survival of esophageal cancer patients. (B) The association between pretreatment CONUT score and cancer-specific survival of esophageal cancer patients.
Sensitivity analysis and publication bias
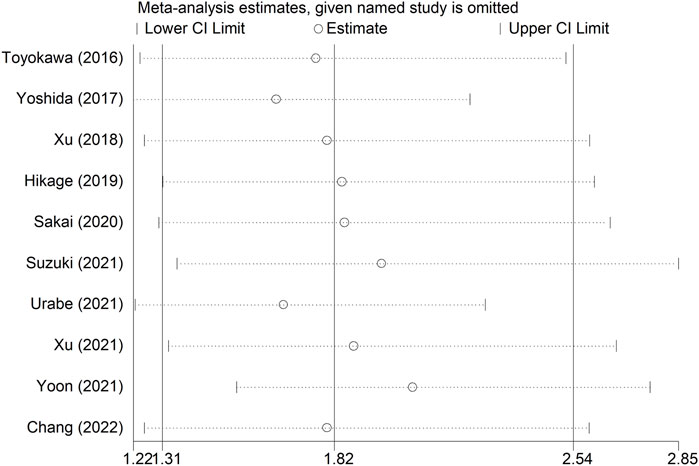
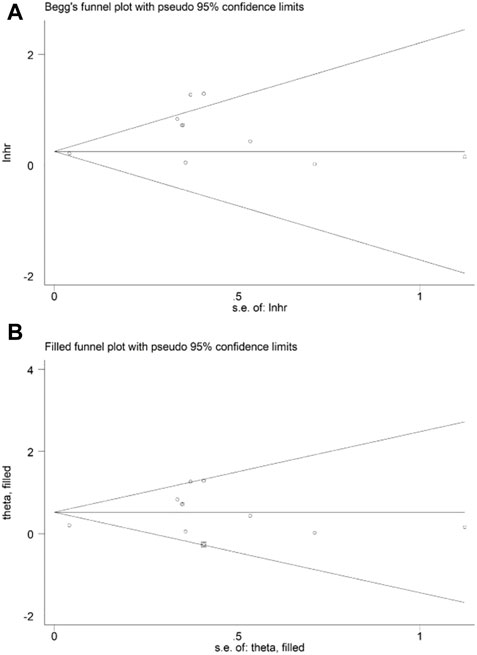
Sensitivity analysis for OS showed that the results of the current meta-analysis were reliable and stable, and none of the included studies showed an obvious impact on the overall results (Figure 4). However, based on the asymmetric Begg’s funnel plot (Figure 5A) and p = 0.050 of Egger’s test, significant publication bias was revealed. Thus, the nonparametric trim-and-fill method was conducted, and only one potentially “unpublished” study was revealed (Figure 5B), but this study did not affect the overall results (filled HR = 1.69, 95% CI: 1.24–2.30, p = 0.001).
Discussion
This meta-analysis showed that the pretreatment CONUT score was associated with the prognosis of esophageal cancer patients, and patients with a higher pretreatment CONUT score experienced poorer OS, PFS and CSS based on current evidence. Therefore, the pretreatment CONUT might contribute to the evaluation of long-term survival and formulation of therapy strategies for esophageal cancer patients. However, because of limitations in this meta-analysis, more high-quality prospective studies are needed to verify the above findings.
The CONUT score consisted of serum albumin concentration, total cholesterol level and total lymphocyte count in peripheral blood. Serum albumin usually reflects the ability of the body to synthesize protein, total cholesterol level reflects the ability of the body to metabolize lipids, and peripheral total lymphocyte count mainly reflects the immune condition of the body [23]. Previous studies have well demonstrated that the nutritional and immune-inflammatory status is closely associated with the development, progression and prognosis of cancers. As mentioned above, the serum and cholesterol and lymphocyte count are good indicators for nutritional and immune-inflammatory status of patients, respectively. Therefore, CONUT score is indicated to be closely related to prognosis of esophageal cancer patients. Overall, the CONUT score is an easier and more objective assessment of nutritional status than the Subjective Global Assessment and the Full Nutritional Assessment [34]. In addition, we deem that the CONUT score might have some advantages over other nutritional indicators, such as the PNI, NRI and GNRI, mentioned above. PNI was calculated as follows: PNI = 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (per mm3) [6], NRI was calculated according to the following formula: NRI = 1.519 × albumin (g/dL) + 41.7 × present weight/usual weight [35], and GNRI was calculated as follows: GNRI = 1.489 × albumin (g/dL) + 41.7 × present weight/ideal weight [36]. First, cholesterol plays an essential role in the progression and prognosis of esophageal cancer patients. It has been verified that the cholesterol level in tumor tissues is higher than that in normal tissues and that the cholesterol metabolite 27-hydroxycholesterol promotes the proliferation and migration of tumor cells [37, 38]. Tao et al. demonstrated that Lysophosphatidylcholine acyltransferase 1 (LPCAT1) reprograms tumor cells cholesterol metabolism in esophageal cancer and could be applied as a potential treatment target against esophageal carcinoma [39]. Furthermore, total cholesterol level was reported as a prognostic factor in esophageal cancer patients [40]. Thus, the cholesterol level in the peripheral blood, reflecting the condition of cholesterol metabolism to some extent, contributes to the evaluation of tumor progression and prognosis. In addition, the effect of body weight on the prognosis of cancer patients is polarizing. In detail, both overweight and low body weight are significantly associated with poor survival in cancer patients [41, 42]. Furthermore, the muscle content could objectively reflect the nutritional status of the body, and several indices, such as the skeletal muscle mass index (SMI), are reported to have high prognostic value in esophageal cancer [43, 44]. However, they are calculated from CT images in a relatively complicated way, which limits their application in clinics.
The prognostic role of the pretreatment CONUT score has been verified in several types of cancers. Ma et al. included seven studies with 2,294 patients and showed that an elevated CONUT score was related to poor OS (HR = 1.56, p = 0.007) in pancreatic cancer [14]. In addition, Takagi et al. demonstrated that a high CONUT score was related to poor OS (HR = 1.97, p < 0.001), CSS (HR = 3.64, p < 0.001) and PFS (HR = 1.68, p = 0.001) after reviewing 2,601 participants [13]. Furthermore, the association between the CONUT score and the prognosis of lung cancer patients was also identified in a meta-analysis by Zhang et al. [15]. Our meta-analysis further verified the predictive role of the CONUT score for the long-term survival of esophageal cancer patients.
There are still several fields about the prognostic value of the CONUT score in esophageal cancer that need further investigation. First, only the relationship between pretreatment CONUT score and survival was identified in most relevant studies. The posttreatment CONUT score and changes in the CONUT score during antitumor treatment might also contribute to the prediction of the therapeutic effect and long-term survival of esophageal cancer. In addition, it is necessary to identify the clinical value of moderately lowering the CONUT score before or during antitumor treatment. For example, whether reducing CONUT scores by consuming cholesterol-high foods and supplementing with albumin could improve the therapeutic effect to antitumor therapy remains unclear. Furthermore, it is also worth investigating whether increasing lymphocyte count before and during the anti-tumor treatment can improve patient outcomes.
There are several limitations in this meta-analysis. First, all included studies were retrospective, and the overall sample size was relatively small. Thus, some bias might exist and should be considered. Second, all patients were from Asian countries, which might limit the generalizability of our conclusions. Third, due to the lack of original data, we were unable to conduct subgroup analysis based on other important parameters, such as TNM stage and age.
Conclusion
The pretreatment CONUT score was predictive of worse prognosis in esophageal cancer, and patients with a higher CONUT score showed worse survival. However, more high-quality prospective studies are still needed to verify our findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CH designed this meta-analysis. JL and PC performed the literature search and selection, collected data and wrote the paper. JL and JW performed the statistical analysis and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Research project of Sichuan Provincial Health Commission (20PJ248).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Siegel, RL, Miller, KD, Fuchs, HE, and Jemal, A. Cancer statistics, 2022. CA: a Cancer J clinicians (2022) 72(1):7–33. doi:10.3322/caac.21708
2. Xia, C, Dong, X, Li, H, Cao, M, Sun, D, He, S, et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin Med J (2022) 135(5):584–90. doi:10.1097/CM9.0000000000002108
3. Bray, F, Ferlay, J, Soerjomataram, I, Siegel, RL, Torre, LA, and Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer J clinicians (2018) 68(6):394–424. doi:10.3322/caac.21492
4. Hursting, SD, and Berger, NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol : official J Am Soc Clin Oncol (2010) 28(26):4058–65. doi:10.1200/JCO.2010.27.9935
5. Norman, K, Pichard, C, Lochs, H, and Pirlich, M. Prognostic impact of disease-related malnutrition. Clin Nutr (Edinburgh, Scotland) (2008) 27(1):5–15. doi:10.1016/j.clnu.2007.10.007
6. Liao, G, Zhao, Z, Yang, H, Chen, M, and Li, X. Can prognostic nutritional index be a prediction factor in esophageal cancer?: A meta-analysis. Nutr Cancer (2020) 72(2):187–93. doi:10.1080/01635581.2019.1631859
7. Ma, LX, Taylor, K, Espin-Garcia, O, Anconina, R, Suzuki, C, Allen, MJ, et al. Prognostic significance of nutritional markers in metastatic gastric and esophageal adenocarcinoma. Cancer Med (2021) 10(1):199–207. doi:10.1002/cam4.3604
8. Yu, J, Zhang, W, Wang, C, and Hu, Y. The prognostic value of pretreatment geriatric nutritional risk index in esophageal cancer: A meta-analysis. Nutr Cancer (2022) 74(9):3202–10. doi:10.1080/01635581.2022.2069273
9. Wang, Y, Chen, L, Wu, Y, Li, P, and Che, G. The prognostic value of modified Glasgow prognostic score in patients with esophageal squamous cell cancer: A meta-analysis. Nutr Cancer (2020) 72(7):1146–54. doi:10.1080/01635581.2019.1677925
10. López-Larramona, G, Lucendo, AJ, and Tenías, JM. Association between nutritional screening via the Controlling Nutritional Status index and bone mineral density in chronic liver disease of various etiologies. Hepatol Res : official J Jpn Soc Hepatol (2015) 45(6):618–28. doi:10.1111/hepr.12395
11. Iseki, Y, Shibutani, M, Maeda, K, Nagahara, H, Ohtani, H, Sugano, K, et al. Impact of the preoperative controlling nutritional status (CONUT) score on the survival after curative surgery for colorectal cancer. PloS one (2015) 10(7):e0132488. doi:10.1371/journal.pone.0132488
12. Yoshida, N, Baba, Y, Shigaki, H, Harada, K, Iwatsuki, M, Kurashige, J, et al. Preoperative nutritional assessment by controlling nutritional status (CONUT) is useful to estimate postoperative morbidity after esophagectomy for esophageal cancer. World J Surg (2016) 40(8):1910–7. doi:10.1007/s00268-016-3549-3
13. Takagi, K, Buettner, S, and Ijzermans, JNM. Prognostic significance of the controlling nutritional status (CONUT) score in patients with colorectal cancer: A systematic review and meta-analysis. Int J Surg (London, England) (2020) 78:91–6. doi:10.1016/j.ijsu.2020.04.046
14. Ma, X, Zou, W, and Sun, Y. Prognostic value of pretreatment controlling nutritional status score for patients with pancreatic cancer: A meta-analysis. Front Oncol (2021) 11:770894. doi:10.3389/fonc.2021.770894
15. Zhang, C, Li, XK, Cong, ZZ, Zheng, C, Luo, C, Xie, K, et al. Controlling nutritional status is a prognostic factor for patients with lung cancer: A systematic review and meta-analysis. Ann Palliat Med (2021) 10(4):3896–905. doi:10.21037/apm-20-2328
16. Peng, L, Meng, C, Li, J, You, C, Du, Y, Xiong, W, et al. The prognostic significance of controlling nutritional status (CONUT) score for surgically treated renal cell cancer and upper urinary tract urothelial cancer: A systematic review and meta-analysis. Eur J Clin Nutr (2022) 76(6):801–10. doi:10.1038/s41430-021-01014-0
17. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clinical research ed) (2021) 372:790–9. doi:10.1016/j.rec.2021.07.010
18. Takagi, K, Buettner, S, Ijzermans, JNM, and Wijnhoven, BPL. Systematic review on the controlling nutritional status (CONUT) score in patients undergoing esophagectomy for esophageal cancer. Anticancer Res (2020) 40(10):5343–9. doi:10.21873/anticanres.14541
19. Wang, Y, Li, J, Chang, S, Dong, Y, and Che, G. Risk and influencing factors for subsequent primary lung cancer after treatment of breast cancer: A systematic review and two meta-analyses based on four million cases. J Thorac Oncol : official Publ Int Assoc Study Lung Cancer (2021) 16(11):1893–908. doi:10.1016/j.jtho.2021.07.001
20. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics (1994) 50(4):1088–101. doi:10.2307/2533446
21. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed) (1997) 315(7109):629–34. doi:10.1136/bmj.315.7109.629
22. Wang, Y, Huang, D, Xu, WY, Wang, YW, and Che, GW. Prognostic value of pretreatment lymphocyte-to-monocyte ratio in non-small cell lung cancer: A meta-analysis. Oncol Res Treat (2019) 42(10):523–31. doi:10.1159/000501726
23. Toyokawa, T, Kubo, N, Tamura, T, Sakurai, K, Amano, R, Tanaka, H, et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: Results from a retrospective study. BMC cancer (2016) 16(1):722. doi:10.1186/s12885-016-2696-0
24. Yoshida, N, Harada, K, Baba, Y, Kosumi, K, Iwatsuki, M, Kinoshita, K, et al. Preoperative controlling nutritional status (CONUT) is useful to estimate the prognosis after esophagectomy for esophageal cancer. Langenbeck's Arch Surg (2017) 402(2):333–41. doi:10.1007/s00423-017-1553-1
25. Hirahara, N, Matsubara, T, Hayashi, H, Takai, K, Nakada, S, and Tajima, Y. Prognostic importance of controlling nutritional status in patients undergoing curative thoracoscopic esophagectomy for esophageal cancer. Am J Ther (2018) 25(5):E524–E532. doi:10.1097/MJT.0000000000000414
26. Xu, J. Analysis of preoperative nutritional data and prognostic model of esophageal squamous cell carcinoma. Doctor. Hangzhou, China: Zhejiang University (2018).
27. Hikage, M, Taniyama, Y, Sakurai, T, Sato, C, Takaya, K, Okamoto, H, et al. The influence of the perioperative nutritional status on the survival outcomes for esophageal cancer patients with neoadjuvant chemotherapy. Ann Surg Oncol (2019) 26(13):4744–53. doi:10.1245/s10434-019-07742-9
28. Sakai, M, Sohda, M, Saito, H, Ubukata, Y, Nakazawa, N, Kuriyama, K, et al. Comparative analysis of immunoinflammatory and nutritional measures in surgically resected esophageal cancer: A single-center retrospective study. vivo (Athens, Greece) (2020) 34(2):881–7. doi:10.21873/invivo.11853
29. Suzuki, T, Furukawa, K, Funasaka, K, Ishikawa, E, Sawada, T, Maeda, K, et al. Long-term prognostic predictors of esophageal squamous cell carcinoma potentially indicated for endoscopic submucosal dissection. Digestion (2021) 102(4):563–71. doi:10.1159/000510091
30. Urabe, M, Ueno, M, Ogawa, Y, Yago, A, Shimoyama, H, Honda, A, et al. Comparative analysis of the prognostic utility of preoperative nutritional parameters in patients with resectable esophageal carcinoma. Gen Thorac Cardiovasc Surg (2021) 69(2):326–35. doi:10.1007/s11748-020-01555-4
31. Xu, D. Inflammatory and nutritional indicators and circRNA in the prognosis of esophageal cancer. Nanjing, China: Nanjing Medical University (2021). 硕士.
32. Yoon, JP, Nam, JS, Abidin, M, Kim, SO, Lee, EH, Choi, IC, et al. Comparison of preoperative nutritional indexes for outcomes after primary esophageal surgery for esophageal squamous cell carcinoma. Nutrients (2021) 13(11):4086. doi:10.3390/nu13114086
33. Chang, L, Cheng, Q, Ma, Y, Wu, C, Zhang, X, Ma, Q, et al. Prognostic effect of the controlling nutritional status score in patients with esophageal cancer treated with immune checkpoint inhibitor. J Immunother (2022) 45:415–22. doi:10.1097/CJI.0000000000000438
34. Tsunematsu, M, Haruki, K, Fujiwara, Y, Furukawa, K, Onda, S, Matsumoto, M, et al. Preoperative controlling nutritional status (CONUT) score predicts long-term outcomes in patients with non-B non-C hepatocellular carcinoma after curative hepatic resection. Langenbeck's Arch Surg (2021) 406(1):99–107. doi:10.1007/s00423-020-01987-9
35. Poulia, KA, Yannakoulia, M, Karageorgou, D, Gamaletsou, M, Panagiotakos, DB, Sipsas, NV, et al. Evaluation of the efficacy of six nutritional screening tools to predict malnutrition in the elderly. Clin Nutr (Edinburgh, Scotland) (2012) 31(3):378–85. doi:10.1016/j.clnu.2011.11.017
36. Bouillanne, O, Morineau, G, Dupont, C, Coulombel, I, Vincent, JP, Nicolis, I, et al. Geriatric nutritional risk index: A new index for evaluating at-risk elderly medical patients. Am J Clin Nutr (2005) 82(4):777–83. doi:10.1093/ajcn/82.4.777
37. Loosen, SH, Kostev, K, Luedde, M, Luedde, T, and Roderburg, C. Low blood levels of high-density lipoprotein (HDL) cholesterol are positively associated with cancer. J Cancer Res Clin Oncol (2022) 148(11):3039–46. doi:10.1007/s00432-021-03867-1
38. Tang, Q, Liang, B, Zhang, L, Li, X, Li, H, Jing, W, et al. Enhanced CHOLESTEROL biosynthesis promotes breast cancer metastasis via modulating CCDC25 expression and neutrophil extracellular traps formation. Scientific Rep (2022) 12(1):17350. doi:10.1038/s41598-022-22410-x
39. Tao, M, Luo, J, Gu, T, Yu, X, Song, Z, Jun, Y, et al. LPCAT1 reprogramming cholesterol metabolism promotes the progression of esophageal squamous cell carcinoma. Cell Death Dis (2021) 12(9):845. doi:10.1038/s41419-021-04132-6
40. Li, Y, Luo, H, Ye, B, Zhang, K, Liu, C, Zu, R, et al. Prognostic value of nutritional and inflammatory indicators in females with esophageal squamous cell cancer: A propensity score matching study. Front Genet (2022) 13:1026685. doi:10.3389/fgene.2022.1026685
41. Harborg, S, Zachariae, R, Olsen, J, Johannsen, M, Cronin-Fenton, D, Bøggild, H, et al. Overweight and prognosis in triple-negative breast cancer patients: A systematic review and meta-analysis. NPJ breast cancer (2021) 7(1):119. doi:10.1038/s41523-021-00325-6
42. Simillis, C, Taylor, B, Ahmad, A, Lal, N, Afxentiou, T, Powar, MP, et al. A systematic review and meta-analysis assessing the impact of body mass index on long-term survival outcomes after surgery for colorectal cancer. Eur J Cancer (2022) 172:237–51. doi:10.1016/j.ejca.2022.05.020
43. Yao, L, Wang, L, Yin, Y, Che, G, and Yang, M. Prognostic value of pretreatment skeletal muscle mass index in esophageal cancer patients: A meta-analysis. Nutr Cancer (2022) 74(10):3592–600. doi:10.1080/01635581.2022.2088814
44. Nishimura, E, Kawakubo, H, Matsuda, S, Fukuda, K, Nakamura, R, and Kitagawa, Y. Long-term variation in psoas muscle mass index is affected by short-term loss after esophagectomy in survivors of esophageal cancer. Dis esophagus : official J Int Soc Dis Esophagus (2022) 36:doac053. doi:10.1093/dote/doac053
Keywords: meta-analysis, prognosis, esophageal cancer, pretreatment, Controlling Nutritional Status score
Citation: Lv J, Chen P, Wu J and Hu C (2023) Prognostic value of pretreatment Controlling Nutritional Status score in esophageal cancer: a meta-analysis. Pathol. Oncol. Res. 29:1611221. doi: 10.3389/pore.2023.1611221
Received: 02 April 2023; Accepted: 14 June 2023;
Published: 27 June 2023.
Edited by:
Andrea Ladányi, National Institute of Oncology (NIO), HungaryCopyright © 2023 Lv, Chen, Wu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caihong Hu, NTIzMTYxODg1QHFxLmNvbQ==
 Jing Lv
Jing Lv Peirui Chen
Peirui Chen