- 1Medical Oncology Division 1, Clinical Oncology Center, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
- 2Department of Dermatology and Venereology, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
Endocrine therapy has played an essential role in hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) breast cancer. With the continuous development of endocrine targeting drugs, especially the emergence of selective cyclin-dependent kinase (CDK4/6) inhibitors, the overall survival time in patients with HR+HER2− advanced breast cancer has been greatly improved. Their adverse reactions also need more attention in response to the climbing number of CDK4/6 inhibitors. The common side effects of CDK4/6 inhibitors were hematological toxicity, diarrhea, and liver function damage. Skin toxicity related to CDK4/6 inhibitors was rare. We describe herein our preliminary observation of one HR+HER2− advanced metastatic breast cancer patient diagnosed with vitiligo-like lesions after 10 months of taking Palbociclib. Hoping to share our experience to increase the clinician awareness of this unusual adverse and contribute to the information in the literature.
Background
Breast cancer is women’s most common malignant tumor globally [1]. Endocrine therapy is a reasonably necessary treatment for HR+ breast cancer. In recent years, with the emergence and widespread application of endocrine-targeted drugs, treatment strategies have resulted in revolutionary changes, further improving patients’ overall survival time and quality of life. CDK4/6 inhibitors are one of the most representative stars. Combination with aromatase inhibitors (AIs) or Fulvestrant has become the preferred first-line treatment for HR+HER2- advanced breast cancer patients [2, 3]. Currently, three CDK4/6 inhibitors worldwide used in the world: Ribociclib, Palbociclib, and Abemaciclib [4, 5]. The most common adverse events (AEs) consist of neutropenia, leukopenia, anemia, fatigue, liver dysfunction, and diarrhea. What’s more, treatment with CDK 4/6 inhibitors also be associated with several potential dermatologic toxicities, including alopecia, which is the most frequent [6]. Vitiligo-like lesions induced by CDK4/6 inhibitors are rare, although some case reports have described vitiligo-like lesions caused by Ribociclib [7, 8].
Here we describe a rare case of vitiligo-like lesions developing after treatment with Palbociclib, a CDK4/6 inhibitor. This 58-year-old woman did not experience any discomfort and insisted on taking the drug for further use of the regimen. We adopted the “watch and wait” strategy, and her progression-free survival time has exceeded 24 months. Intending to share our experience to raise awareness among clinicians and contribute to the literature, we present the following information based on the CARE reporting checklist [9].
Case presentation
Ethics
Written informed consent was obtained from the participant for the publication of any potentially identifiable images or data included in this article.
A 58-year-old female patient was diagnosed with right-side breast invasive lobular carcinomas through imaging examinations and pathological assessment in November. 2020. Ultrasonography revealed a 9*8 mm mass in the seven o’clock direction, with BI-RADS-Us stage IVb. Immunohistochemistry showed that the tumor cells were estrogen receptor/progesterone receptor-positive, and HER2-, Ki-67 5%–10% positive, the clinical stage is cT1N0M1, stage IV, the molecular subtype is Luminal A, with multiple bone metastases at the same time.
As she was in a premenopausal state at that time, according to the guidelines, she started Palbociclib and letrozole as the first-line therapy after receiving ovarian ablation in January 2021.
Ten months later, the patient presented with multiple irregularly, well-demarcated, hypopigmented lesions over both axillary fossa that gradually spread to the arms, legs and lower back without pruritus or erythema. She did not have any other comorbidities, nor did she have a family history of vitiligo, hyperthyroidism, or systemic lupus erythematosus. A dermatological examination revealed multiple depigmented macules of varying sizes over the hands, forearms, thighs, feet, fossa axillaries, and lower back (Figure 1). A wood lamp examination showed blue-white patches with clear boundaries and increased pigmentation at the edges of white patches. Skin biopsies were taken from depigmented macules; the histological pathology shows the complete absence of melanocytes and melanin, in addition to a mild infiltration of inflammatory cells around the blood vessels (Figure 2). Based on these examination results, non-segmental vitiligo of grade 2 was diagnosed, covering approximately 6% of the body surface area (BSA). Since she did not experience any discomfort, she refused treatment with medicine or laser therapy. She continues to take Palbociclib and letrozole to date.
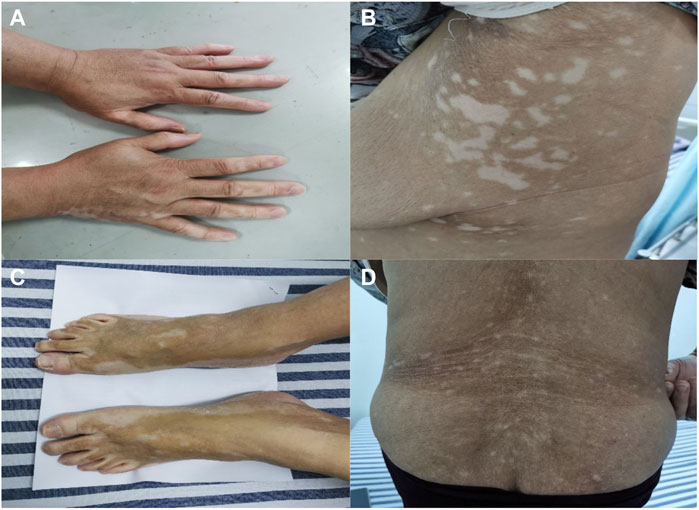
FIGURE 1. Clinical images. The non-segmental/bilateral depigmented macules over the hands (A), fossa axillaries (B), and feet (C). Vitiligo-like lesions over the lower back (D). Multiple irregularly, well-demarcated, hypopigmented lesions surrounded by normal skin.
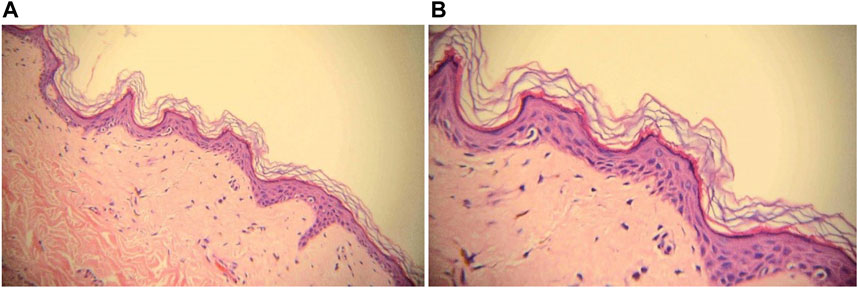
FIGURE 2. Biopsy with H&E staining of depigmented macule, ×20 magnification (A) and ×40 magnification (B). Melanocytes and melanin granules in the epidermis of the leukoplakia area were absent.
As of January 2023, the tumor and vitiligo are stable, and the progression-free survival time has exceeded 24 months.
Discussion
Paloma-2 [10], a randomized controlled double-blind phase III clinical study investigating the efficacy and safety of Palbociclib in combination with letrozole in patients with primary HR+HER2− advanced breast cancer. A total of 666 patients were enrolled in the study. Median progression-free survival was 24.8 months for Palbociclib -letrozole versus 14.5 months for placebo-letrozole (hazard ratio, 0.58; 95% CI, 0.46 to 0.72; p < 0.001). The most common treatment-related adverse events (AEs) of grade 3 or 4 were neutropenia, leukopenia, anemia, and fatigue. Dermatologic AEs reported in this article included alopecia (Any grade 32.9%), rash (Any grade 17.8, Grade 3 0.9%), and dry skin (Any grade 32.9%). No dermatologic AEs of vitiligo-like lesions were reported.
Vitiligo is an acquired pigmentary autoimmune disorder consisting of developing hypopigmented macules due to the selective loss of melanocytes. Possible mechanisms for the development of vitiligo include autoimmunity, oxidative stress, melanocyte self-destruction, and genetic predisposition [7, 11]. It’s not life-threatening. Nevertheless, it affects patients’ psychosocial health, interpersonal communication, and health-related quality of life [12]. In patients treated with immune checkpoint inhibitors, vitiligo-like lesions caused by antineoplastic drugs are occasionally reported. We were also surprised to find occasional reports of vitiligo-like lesions associated with B-cell leukemia/lymphoma-2 (Bcl-2) inhibitor Venetoclax [13].
Vitiligo-like lesions induced by cyclin-dependent kinase 4/6 (CDK4/6) inhibitors are rare, and the pathogenesis of vitiligo-like lesions induced by CDK4/6 inhibitors is also poorly understood. Zhou [14] hypothesized that CDK inhibitors can block the cell cycle and deregulate the expression of small ubiquitin-like modulators expression in keratinocytes, eventually resulting in the occurrence of vitiligo.
To our knowledge, only three cases of Pabociclib-induced vitiligo-like lesions have been reported in the literature. The data on the previous reports were summarized data are enumerated in Table 1. Sollena [15], on behalf of the European Network for Cutaneous ADverse event to Oncologic drugs (ENCADO) group, first reported two cases of vitiligo-like lesions after using Palbociclib in the treatment of 16 patients with metastatic breast cancer in six European university-based centers. Both two patients experienced cutaneous pruritus. Patient No. 1 was affected with vitiligo-like lesions in 60% of BSA after taking 8 months of Palbociclib and Fulvestrant was treated with oral corticosteroids and calcineurin inhibitors, the outcome of dermatologic treatment is PR, the lesions reached an improvement and the area reduced. Patient No. 2 found vitiligo-like lesions in 12 months of using Palbociclib and Letrozole was treated with topical oral corticosteroids. No. 3 is a patient who appeared hypopigmented lesions after 1 month of changing from Ribociclib to Pabociclib. At the same time, she decided to refuse to take any treatment, and the cutaneous lesions were stable within a 4-month follow-up [16].
Our patient was diagnosed with vitiligo after 10 months of Palbociclib and letrozole treatment. Her occurrence time of vitiligo-like lesions is approximately consistent with the patients’ described in Sollena’s study. As she did not experience any discomfort, such as inflammation or pruritus, she does not agree to any additional medication. Tumors and vitiligo were observed to remain stable for up to now, more than 14 months. We have reason to suspect that these vitiligo-like skin lesions are related to Palbociclib in the absence of prior evidence that letrozole may cause specific hypopigmented macules. We need to be vigilant about this rarely-seen adverse; counselling patients regarding the possibility of vitiligo¬like lesions prior to initiation of Palbociclib may help lessen the impact of this adverse event on the patient’s mental health and quality of life.
Conclusion
Our case adds to the knowledge gained from the existing case reports in the literature. Firstly, vitiligo-like skin lesions can be encountered late in Palbociclib therapy. Second, the range of vitiligo-like skin lesions does not show remission during an intermission in treatment, which provides evidence that these lesions induced by Palbociclib are irreversible. Future large-scale clinical studies are needed to provide more evidence for this hypothesis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the participant for the publication of any potentially identifiable images or data included in this article.
Author contributions
GW supplied the study conception and design, SG researched the literature and writing, and YH supplied the discussion and revision. All authors contributed to the article and approved the submitted version.
Funding
The current study was supported by grants from the Guangxi Zhuang Autonomous Region Natural Science Foundation of (grant no. 2022GXNSFBA035493).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Siegel, RL, Miller, KD, Fuchs, HE, and Jemal, A. Cancer statistics, 2022. CA-Cancer J Clin (2022) 72:7–33. doi:10.3322/caac.21708
2. Hortobagyi, GN, Stemmer, SM, Burris, HA, Yap, YS, Sonke, GS, Hart, L, et al. Overall survival with Ribociclib plus letrozole in advanced breast cancer. New Engl J Med (2022) 386:942–50. doi:10.1056/NEJMoa2114663
3. Sledge, GJ, Toi, M, Neven, P, Sohn, J, Inoue, K, Pivot, X, et al. The effect of Abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: A randomized clinical trial. Jama Oncol (2020) 6:116–24. doi:10.1001/jamaoncol.2019.4782
4. Spring, LM, Wander, SA, Andre, F, Moy, B, Turner, NC, and Bardia, A. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: Past, present, and future. Lancet (2020) 395:817–27. doi:10.1016/S0140-6736(20)30165-3
5. Giuliano, M, Schettini, F, Rognoni, C, Milani, M, Jerusalem, G, Bachelot, T, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: A systematic review and network meta-analysis. Lancet Oncol (2019) 20:1360–9. doi:10.1016/S1470-2045(19)30420-6
6. Silvestri, M, Cristaudo, A, Morrone, A, Messina, C, Bennardo, L, Nisticò, SP, et al. Emerging skin toxicities in patients with breast cancer treated with new cyclin-dependent kinase 4/6 inhibitors: A systematic review. Drug Safety (2021) 44:725–32. doi:10.1007/s40264-021-01071-1
7. Silvestre, TN, Aguilar, MA, Echarri, GM, Tabbara, CS, Román, SJ, and Gruber, VF. Ribociclib-induced vitiligo: A case report. Dermatol Pract Conce (2022) 12:e2022045. doi:10.5826/dpc.1202a45
8. Chan, OB, Su, JC, Yazdabadi, A, and Chan, A. Drug induced vitiligo-like depigmentation from a CDK 4/6 inhibitor. Asia-Pac J Clin Onco (2022) 18:e154–e156. doi:10.1111/ajco.13585
9. Gagnier, JJ, Kienle, G, Altman, DG, Moher, D, Sox, H, Riley, D, et al. The CARE guidelines: Consensus-based clinical case reporting guideline development. BMJ Case Rep (2013) 2:38–43. doi:10.7453/gahmj.2013.008
10. Finn, RS, Martin, M, Rugo, HS, Jones, S, Im, SA, Gelmon, K, et al. Palbociclib and letrozole in advanced breast cancer. New Engl J Med (2016) 375:1925–36. doi:10.1056/NEJMoa1607303
11. Anjaneyan, G, Keechilat, P, Duraisamy, P, and Eapen, M. Ribociclib-induced extensive vitiligo-like lesions: Possible pathomechanisms with clinical, dermoscopic and histological correlation. BMJ Case Rep (2022) 15:e248782. doi:10.1136/bcr-2022-248782
12. Papadopoulos, L, Bor, R, and Legg, C. Coping with the disfiguring effects of vitiligo: A preliminary investigation into the effects of cognitive-behavioural therapy. Br J Med Psychol (1999) 72(3):385–96. doi:10.1348/000711299160077
13. Abdeen, M, Vusqa, UT, Asawa, P, Felton, K, Rinchuse, D, Khan, C, et al. Venetoclax-induced vitiligo in a patient with chronic lymphocytic leukemia. Anti-Cancer Drug (2022) 33:1167–70. doi:10.1097/CAD.0000000000001350
14. Zhou, M, Lin, F, Xu, W, Jin, R, and Xu, A. Decreased SUMOylation of the retinoblastoma protein in keratinocytes during the pathogenesis of vitiligo. Mol Med Rep (2018) 18:3469–75. doi:10.3892/mmr.2018.9299
15. Sollena, P, Nikolaou, V, Soupos, N, Kotteas, E, Voudouri, D, Stratigos, AJ, et al. Vitiligo-like lesions in patients with advanced breast cancer treated with cycline-dependent kinases 4 and 6 inhibitors. Breast Cancer Res Treat (2021) 185:247–53. doi:10.1007/s10549-020-05914-w
Keywords: breast cancer, CDK4/6 inhibitors, Palbociclib, adverse events, vitiligo-like lesions
Citation: Gao S, Wei G and Hao Y (2023) Vitiligo-like lesions induced by cyclin-dependent kinase 4/6 inhibitor Palbociclib: a case report and literature review. Pathol. Oncol. Res. 29:1611115. doi: 10.3389/pore.2023.1611115
Received: 14 February 2023; Accepted: 18 May 2023;
Published: 06 July 2023.
Edited by:
Aniko Maraz, University of Szeged, HungaryCopyright © 2023 Gao, Wei and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanjing Wei, NjQxNjM0NTQwQHFxLmNvbQ==
 Shan Gao1
Shan Gao1 Guanjing Wei
Guanjing Wei