- 1Department of Urology, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, China
- 2Department of Pathology, Binzhou Medical University Hospital, Binzhou, China
- 3Department of Imaging, Binzhou Medical University Hospital, Binzhou, China
- 4Department of Imaging, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, China
- 5Department of Pathology, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, China
- 6Department of Gastroenterology, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, China
Background: Composite lymphomas involving B-cell and T-cell lymphomas is very rare.
Case presentation: We reported a 63-year-old gentleman with composite chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL). The patient was admitted to our hospital due to abdominal pain, and was diagnosed with CLL/SLL after bone marrow (BM) biopsy, BM aspiration, and flow cytometry. Two weeks later, he was diagnosed with MEITL based on pathological analysis after intestine excision. Next gene sequencing (NGS) findings identified two hotspot mutation sites (STAT5B and DNMT3A) closely related with the pathogenesis of CLL/SLL and MEILT. Additionally, BCOR mutation was only detected in the CLL/SLL area. The likely pathogenic mutations of CLL were SETD2, NOTCH1, SF3B1, and PTPN11, while the likely pathogenic mutations related with the MEILT were TET2 and ZRSR2. Mutations of GATA3, PLCG2, and FAT1 were identified in both CLL/SLL and MEITL areas, but the clinical significance was unknown. Finally, the patient died in the 12-month follow-up after surgery.
Conclusion: We report a rare case of composite CLL/SLL and MEITL that highlights the importance of careful inspection of hematologic neoplasms. We also present the results of NGS of different gene mutations in CLL and MEITL tissues.
Introduction
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is a typical indolent non-Hodgkin lymphoma (NHL) characterized by accumulation of malignant B cells in bone marrow (BM), peripheral blood and lymph nodes. Richter’s syndrome refers to high-grade NHL or Hodgkin lymphoma (HL) in patients with CLL/SLL. Approximately 2%–8% of patients with CLL/SLL would develop diffuse large B cell lymphoma (DLBCL), and less than 1% would present classic HL (1). In rare cases, CLL/SLL patients present T-cell malignancies with an incidence of approximately 1%, which is usually in the anaplastic large cell type or with a cytotoxic phenotype (2). Monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) is a rare primary intestinal T-cell lymphoma formerly known as type 2 enteropathy-associated T-cell lymphoma (EATL), resulting in very poor prognosis. In this study, we reported an extremely rare case presenting simultaneous CLL/SLL and MEITL.
Case presentation
A 63-year-old gentleman was referred to our hospital due to needle-like pain in the upper abdomen for at least 15 days on November 18, 2020. The pain was transient and was relieved shortly, but he showed serious pain and vomiting occasionally. Gastroscopy showed reflux esophagitis and chronic atrophic gastritis. Doppler ultrasonography of the abdomen showed no abnormalities. Routine blood examination results were as follows: white blood cell, 17.9 × 109/L (normal range: 4–10 × 109/L); lymphocyte ratio, 62.5% (normal range: 20%–40%); lymphocyte, 11.0 × 109/L (normal range: 1–3 × 109/L); erythrocyte, 4.7 × 1012/L (normal range: 3.5–5.5 × 1012/L); hemoglobin, 87 g/L (normal range: 110–160 g/L); mean corpuscular volume (MCV), 67 fL (normal range: 82–92 fl); corpuscular hemoglobin (MCH), 19 pg (normal range: 27–31 pg); mean corpuscular hemoglobin concentration (MCHC), 284 g/L (normal range: 320–360 g/L); and ferritin, 8.21 ng/ml (normal range: ≥ 20 ng/ml). Upon physical examination, the patient showed bilateral inguinal lymphadenectasis. A smoking history was reported by himself with a duration of about 20 years (50 cigarettes per day). Nowadays, the patient does not smoke for 20 years. He had a history of drinking alcohol (about 150 g per day) for 40 years.
BM biopsy showed a markedly hypercellular marrow (85%), which was diffusely involved by a small lymphocytic infiltration (80%) (Figure 1A). The infiltration consisted of numerous small lymphocytes and scattered paraimmunoblasts (Figure 1B). The tumor cells were positive for CD20, CD5, and CD23, and were negative for CD3, CD10, TdT, and cyclin D1 (Figures 1C–E). For the aspirate smear, the majority (80%) were small lymphocytes with coarse chromatin. Peripheral blood smear showed scattered, small to medium sized lymphoma cells with irregular nuclear contour. Flow cytometry on BM aspirate demonstrated the presence of kappa restricted B cells, together with positive staining for CD19, CD20, CD22, CD5, and CD23. No CCND1 gene break or P53 gene deletion was identified by FISH, while no mutation was identified in MYD 88 gene after Sanger sequencing.
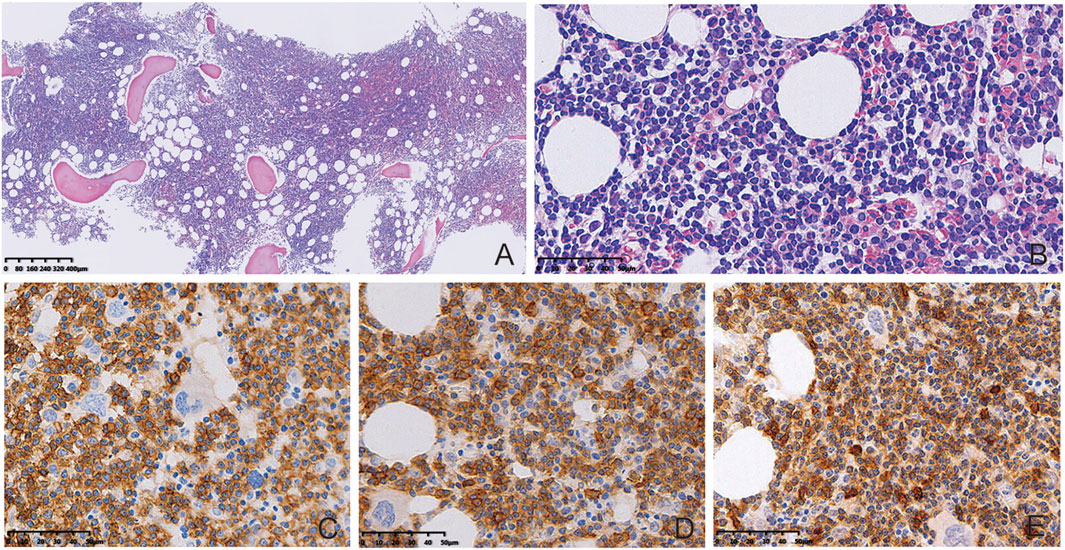
FIGURE 1. BM biopsy results. BM biopsy showed diffuse involvement by a small lymphocytic infiltration ((A), HE, 40×). Presence of numerous small lymphocytes and scattered paraimmunoblasts in the infiltrated tissues ((B), HE, 400×). The tumor cells were positive for CD20 ((C), Enlivision, 400×), CD23 ((D), Enlivision, 400×), and CD5 ((E), Enlivision, 400×).
The patient was then diagnosed with CLL/SLL and microcytic hypochromic anemia simultaneously. Thoracic and abdominal CT showed lymphadenopathy in mediastinum, bilateral neck, axillary area, enterocoelia, retroperitoneal and pelvic cavity, as well as splenomegaly. CT scan indicated partial thickening in the small intestine. Thus, low-stage CLL/SLL was considered, and the patient merely received iron supplement for the treatment. However, the patient still reported persistent needle-like pain in the upper abdomen after treatment. CT enterography showed thickening in the small intestinal wall (Figures 2A–C), together with multiple lymphadenopathies in the abdominal cavity and retroperitoneum. Colonoscopy indicated three polyps with a size of 0.8 cm, 0.7 cm, and 0.4 cm, respectively. Pathological analysis indicated tubular adenoma with low-grade dysplasia. Finally, the patient was discharged with attenuation in the abdominal pain without treatment.
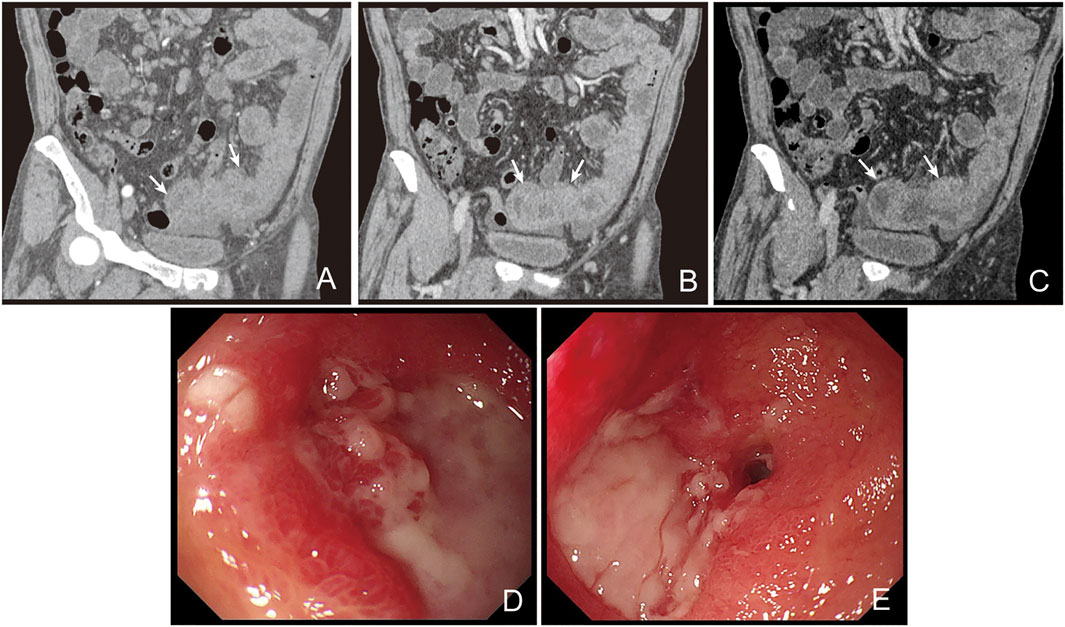
FIGURE 2. CT scan and enteroscope of the patient. CT enterograph of the small intestine showed even thickening in the wall of the small intestine (jejunum) in the pelvic cavity with multiple lymphadenopathy in enterocoelia and retroperitoneum ((A), arterial phase; (B), portal phase; (C), lag phase). Enteroscopic examination showed multiple ulcers in the distal jejunum (D). The ulcers were deeper with irregular margins and were covered with white pus moss. Enteroscopic examination showed stenosis in the jejunum (E).
The patient showed recurrence of abdominal pain lasting for 2 weeks after discharge. Abdominal CT showed increase in the diameter and number of lymph nodes. Enteroscopic examination indicated multiple ulcers in the distal jejunum, which were deeper in site with an irregular margin covering them with white pus moss (Figures 2D,E). Based on the biopsy, inflammatory disease was considered by a general pathologist. Then he received laparoscopic exploration and partial enterectomy after endotracheal intubation and combined intravenous-inhalation anesthesia, which indicated multiple ulcers and narrowing in the small intestine. The intestinal wall was narrow and there was a mass at the position that was about 100 cm from the Treitz. Then a part of the intestine including the narrow lesion and the mass (2.5 cm × 1.0 cm × 0.5 cm) was excised. A narrowing site in the intestinal wall was observed at a distance of 2.5 cm from the mass. There was an ulcer in the lesion with a length and a diameter of 2.0 cm and 0.5 cm, respectively. Intestinal edema was noticed between the mass and the narrowed intestinal wall. The villous architecture was distorted in the mass and the whole layer was diffusely infiltrated by neoplastic lymphocytes. The villi were broadly expanded and infiltrated by the tumor cells, presenting prominent epitheliotropism in the peripheral intestine (Figures 3A–C). The cells were moderate in size with a generous rim of the pale cytoplasm. The nuclei were irregular, and most of them showed finely dispersed chromatin and inconspicuous nucleoli. Partial nuclei showed nuclear fold, prominent nucleoli, and dense chromatin. There was no inflammatory background or necrosis. Additionally, small lymphoid cells were seen around the blood vessels in the intestinal wall (Figure 3D).
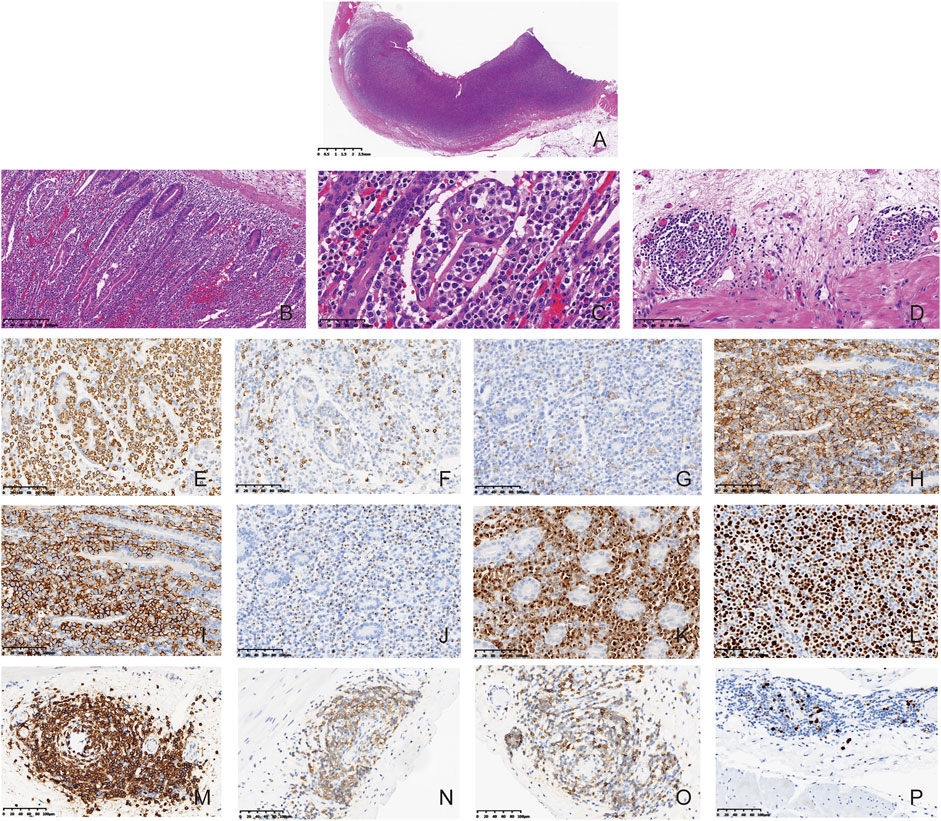
FIGURE 3. Composite CLL/SLL and MEITL. Microscopically, the intestinal wall was diffusely infiltrated by the neoplastic lymphocytes ((A), HE, 8×). The villous architecture was distorted and the neoplastic cells showed prominent epitheliotropism ((B,C), HE, 100×). The cells were generally medium in size with a generous rim of the pale cytoplasm. The nuclei were irregular, and most of them had finely dispersed chromatin and inconspicuous nucleoli. ((C), HE, 400×) CLL/SLL tumor cells were found around the blood vessels in the intestinal wall at the periphery of the tumor ((D), HE, 200×). The MEITL tumor cells in the mass were positive for CD3 ((E), Enlivision, 200×), negative for CD5 (F), Enlivision, 200×) and CD4 ((G), Enlivision, 200×), positive for CD8 ((H), Enlivision, 200×), CD56 ((I), Enlivision, 200×), TIA-1 ((J), Enlivision, 200×) and Granzyme B ((K), Enlivision, 200×). The Ki-67 index of MEITL was in a range of approximately 70%–80% ((L), Enlivision, 200×). The CLL/SLL tumor cells in the mass were positive for CD20 ((M), Enlivision, 200×), CD23 ((N), Enlivision, 200×), and CD5 ((O), Enlivision, 200×). The Ki-67 index of CLL/SLL were about 5%–10% ((P), Enlivision, 200×).
The tumor cells were positive for CD3, CD7, CD8, CD43, CD56, TIA1, Granzyme B, and Bcl-2. In addition, the tumor cells were negative for CD20, CD79a, CD10, Bcl-6, MUM-1, CD23, cyclin D1, CD2, CD4, CD5, and CD30. The Ki-67 index was in a range of 70%–80% (Figures 3E–L). Tumor cells near the blood vessels were positive for CD20, CD79a, CD5, CD23, CD43, and Bcl-2. The Ki-67 index was in a range of 5%–10% (Figures 3M–P). The results were summarized in Table 1. At the focal edge area of the mass, there was mixed immunophenotype expression. The expression of EBV was negative in the tumor mass and the cells in the peripheral vessels. Finally, the patient was diagnosed with composite lymphoma with MEITL and CLL/SLL simultaneously. The peripheral blood and BM were not invaded by MEILT tumor cells based on the aspiration and the BM biopsy, which was confirmed by flow cytometry to the BM and peripheral blood.
There was clonal rearrangement for the IG detection using specific BIOMED-2 primers based on peripheral blood sample. Sanger sequence confirmed that it was IGHV3-33_06. The homologous degree was 100% compared with the germline sequence. PCR-based TCR assay revealed a small monoclonal T-cell population in a background of oligoclonal T-cells.
For the next-generation sequencing (NGS), B- and T-neoplastic cell content was estimated based on morphology, immunohistochemistry (IHC) and IG/TCR gene rearrangement. Two different paraffin blocks, with about 20% neoplastic cells in the HE sections, were selected for IG and TCR gene rearrangements and targeted sequencing. IHC indicated CLL/SLL harboring IG gene rearrangement, and MEILT harboring TCR gene rearrangement. Enrichment for areas of interest was scraped manually when comparing with the HE staining. High-throughput sequencing analysis was performed using haematopoietic and lymphoid specific panels covering the coding sequence (CDS) of 143 Hematological Disease genes through Illumina NextSeq 550 with a mean sequencing depth of 1000× (SINO-US Diagnostics Lab, Tianjin, China) (Supplementary Table S1). Data were analyzed using the bioinformatics pipeline in-house.
According to the NGS results, the pathogenic hotspot mutations of CLL/SLL and MEILT were STAT5B (c.1924A>C, p.N642H) and DNMT3A (c.2678G>A, p.W893*). Additionally, gene mutation on BCOR (c.1005dupC, p.S336Lfs*45) was only detected in the CLL/SLL area. The likely pathogenic mutations of CLL were SETD2 (c.4688delG, p.G1563Afs*2), NOTCH1 (c.7541_7542del, p.P2514Rfs*4), SF3B1 (c.2902-2A>T), and PTPN11 (c.1492C>T, p.R498W). The TET2 (c.4094G>T, p.G1365V) and ZRSR2 (c.1237A>G, p.K413E) mutations were likely pathogenic for the MEILT. Furthermore, mutations on GATA3 (c.124C>T), PLCG2 (c.1311T>G), and FAT1 (c.7130C>T) were detected in both CLL/SLL and MEITL areas, which were classified as variants of uncertain clinical significance (UVS) (Table 2).
For the follow-up, the patients did not receive additional treatment and passed away on January 6, 2022. This study was performed according to the convention of the Declaration of Helsinki, and the study protocols were approved by the Ethics Committee of Qilu Hospital (Qingdao). Written informed consent was obtained from the patient and his guardians.
Discussion
Composite lymphoma is defined as coexistence of two distinct types of NHL or a rare combination of HL and NHL in a single organ or tissue (3), comprising 1%–4% of malignant lymphomas (4). Discordant lymphoma is defined as two distant sites involving two different lymphomas. In cases of sequential occurrence of two different lymphomas, it is known as a secondary lymphoma. Our patient was first diagnosed with CLL/SLL, and then was diagnosed with MEITL 1 month afterwards. The abdominal symptoms were presented at the first visit and the two NHLs were presented in the intestinal wall simultaneously. Therefore, the patient was diagnosed with composite lymphoma with CLL/SLL and MEITL.
In 1992, Strickler et al firstly reported 2 cases with nodal peripheral T cell lymphoma (PTCL) concurrent with CLL/SLL (5). Since then, 36 additional cases had been reported, among which 21 cases showed composite lesions involving both PTCL and CLL/SLL in the same biopsy (6). Among these 38 cases, 22 cases (57.9%) were qualified as PTCL or PTCL nos, including 12 with a cytotoxic phenotype, while 12 cases (31.6%) were ALCLs including 6 ALK-positive, 5 ALK-negative, and 1 ALK status unknown. The other 4 cases (10.5%) showed aggressive EBV-negative natural killer (NK)-cell leukemia (n = 1), EBV-positive nasal NK/T-cell lymphoma (n = 1), T-large granular lymphocyte leukemia (n = 1), and nodal lymphoma with a TFH cell phenotype (n = 1) (6). MEITL is a cytotoxic CD8+ T cell lymphoma, which accounts for the vast majority of primary intestinal T-cell lymphoma in Asia. Most MEITL cases show jejunal and ileal involvement. The neoplastic cells are featured by medium-sized round nuclei with a rim of pale cytoplasm, which usually infiltrate the intestinal epithelium. Our patient presented abdominal pain, and the medium-sized tumor cells infiltrated the intestinal wall with prominent epitheliotropism. Finally, the patient was confirmed with CLL and MEITL based on the immunophenotype analysis.
The patient was misdiagnosed as Inflammatory disease at first based on biopsy by a general pathologist. After reviewing the biopsy slides, we noticed atypical lymphoid cells in the background of necrosis and inflammatory exudation. Some tissues were crashed and the morphology was indistinguishable. Besides, there was no epitheliotropism in the biopsy. These may lead to misdiagnosis based on the biopsy. Furthermore, attention should be given to the morphology of the tissues, in order to avoid misdiagnosis.
In our patient, STAT5B, DNMT3A, GATA3, PLCG2 and FAT1 mutations were identified in both CLL/SLL and MEITL tumor cells. Activating mutations in STAT5B have been identified in a high proportion of MEITL cases (7-9). In a previous study, Diamantopoulos et al showed that the expression of STAT5B was correlated with the presence of EBV and LMP1, which was negatively correlated with the overall survival of the CLL patients (10). Somatic DNMT3A mutations were rarely identified in CLL and MEITL patients, however, low DNMT3A expression was associated with the pathogenesis of more aggressive diseases (11, 12). Trimech et al reported a small series of patients with composite lymphomas consisting of CLL/SLL and angioimmunoblastic T-cell lymphoma (AITL), in which the AITL comprised prominent clear cells with similar mutations consisting of TET2 or DNMT3A alterations (6). DNMT3A mutation was identified in both CLL/SLL and MEITL tissues in our case. As the expression of VAF was low, DNMT3A might be a manifestation of clonal hematopoiesis of indeterminate potential (CHIP) in case of the patient. In addition, TET2 mutation was found in MEITL area rather than the CLL area, which was not frequent in MEITL patients (12). As the expression of VAF was 8.4%, we think TET2 mutation might be a passenger mutation or a manifestation of CHIP in these patients. Mutational analyses suggested that CLL progression on ibrutinib was closely related to the mutations of BTK and/or PLCG2 genes (13). Monica et al reported that 10.3% of fludarabine refractoriness (FR)-CLL cases showed mutations of FAT1 gene that encoded a cadherin-like protein involving in the negative regulation of Wnt signaling. On this basis, FAT1 mutation may play important roles in the development of a high-risk phenotype (14). In our case, PLCG2 and FAT1 mutations were also detected in MEITL areas, and GATA3 mutations were identified in CLL and MEITL. GATA3, PLCG, and FAT mutations showed similar VAFs in CLL and MEITL tissues, which indicated that the mutations in this patient were germline variants.
Mutations of SETD2, NOTCH1, SF3B1, BCOR, and PTPN11 were identified in the CLL/SLL area of our patients. In a previous study, Parker et al. identified recurrent deletions of the SETD2 locus in 3% (8/261) of CLL patients, and they detected mutations of SETD2 in an additional 3.8% of patients (23/602) based on NGS results (15). Their data highlighted SETD2 aberration as a recurrent, early loss-of-function event in CLL pathobiology linked to aggressive diseases (15). However, the alterations in SETD2 gene were more frequent in MEITL as recorded in 93% of Western European cases (16), and in 70% of cases from North America (17). In contrast, Chen et al. only reported two cases with SETD2 mutation in Chinese MEITL patients (8). In our case, SETD2 mutation was found in the CLL/SLL area, not the MEITL area. Mutations in NOTCH1 and SF3B1 were associated with a poor prognosis in CLL/SLL cases (1, 18). The SF3B1 variant showed a very low allele burden and loss of function, which was not likely to be an oncologically relevant variant in this patient. Previously, Richter’s transformation had been considered to be associated with NOTCH1 mutations (1, 19), and BCOR mutation was detected in 6.25% of CLL patients (20). This was the first report on PTPN11 mutation in CLL and ZRSR2 mutation in MEITL. However, the VAF of PTPN11 and ZRSR2 is very low, and further studies are required to investigate the unknown significance.
The following aspects may help to explain the pathology of composite lymphomas. Firstly, there was an underlying chronic immune dysregulation and T-cell stimulation in CLL patients. Certain factors secreted by CLL cells (e.g. inflammatory cytokines), may chronically stimulate normal lymphocytes, which then could lead to neoplastic transformation (21). In addition, studies on cellular immunity in patients with CLL/SLL reported reduced T-cell function with a paradoxical clonal expansion of CD8+ T cells and increased circulating CD8+ T-cell counts (22). This might explain the fact that the majority of T-cell lymphomas in CLL/SLL patients may have a cytotoxic phenotype, which expressed CD8 and/or cytotoxic granule proteins, in contrast to T-cell lymphomas in the general population (2, 6, 23-25). Secondly, composite lymphomas may be transformed from a stem cell with the possibility of developing into B-cell or T-cell lineages under different stimulations. Thirdly, the composited malignancies may not be directly related but may represent a shared genetic predisposition or step-by-step oncogenic potential. In cases with simultaneous diagnosis of CLL/SLL and T-cell lymphoma, it is not possible to determine which is primary. Nevertheless, in the present case, no specific treatment regimen was given to the CLL prior to diagnosis of MEILT. Additionally, both CLL and MEITL harbored STAT5B, DNMT3A, GATA3, PLCG2, and FAT1 mutations. All these suggested that these two lymphomas may be derived from the same progenitor cells, while the different gene mutations might indicate that the progenitor cells received different environmental stimulation.
Conclusion
In summary, we report a rare case of composite CLL/SLL and MEITL that highlights the importance of careful histopathologic inspection and immunophenotypic features of hematologic neoplasms. We also highlight the utilization of NGS in screening different gene mutations in CLL and MEITL tissues.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Qilu Hospital (Qingdao). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant for the publication of this case report.
Author contributions
BZ: Writing, original draft preparation. QL, QJ, and RL: Collected the data. YZ, WC, and HG: Analyzed the data, reviewing, and editing. All authors read the final manuscript and approved the submitted version.
Funding
This work was supported by Natural Science Foundation of Shandong Province (No. ZR2020MC068 to HJ).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely appreciate Professor Xiaoqiu Li (Department of Pathology, Fudan University Shanghai Cancer Center) for the help in the diagnosis of the case. We sincerely appreciate the technician Long Chen (SINO-US Diagnostics Lab, Tianjin, China) and Professor Yuan Tang (Department of Pathology, West China Hospital) for the help in the interpretation of the NGS results.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2022.1610653/full#supplementary-material
Abbreviations
AITL, angioimmunoblastic T-cell lymphoma; BM, bone marrow; BTK, Bruton tyrosine kinase; CHIP, clonal hematopoiesis of indeterminate potential; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; EATL, enteropathy-associated T-cell lymphoma; FR, fludarabine refractoriness; HL, Hodgkin lymphoma; IHC, immunohistochemistry; MCH, corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma; NGS, next-generation sequencing; NHL, non-Hodgkin lymphoma; PLCG2, phospholipase Cγ2; PTCL, peripheral T cell lymphoma.
References
1. Campo, E, Ghia, P, Montserrat, E, Harris, N, Müller-Hermelink, H, Stein, H, et al. Chronic lymphocytic leukaemia/small lymphocytic lymphoma, who classification of tumours of haematopoietic and lymphoid tissues lyon. Lyon: IARC (2017). p. 216–21.
2. Van Der Nest, BM, Leslie, C, Joske, D, Radeski, D, White, R, and Cheah, CY. Peripheral T-cell lymphoma arising in patients with chronic lymphocytic leukemia. Am J Clin Pathol (2019) 152:818–27. doi:10.1093/ajcp/aqz109
3.National cancer institute sponsored study of classifications of non-hodgkin's lymphomas: summary and description of a working formulation for clinical usage. The non-hodgkin's lymphoma pathologic classification project. Cancer (1982) 49:2112–35. doi:10.1002/1097-0142(19820515)49:10<2112:aid-cncr2820491024>3.0.co;2-2
4. Thirumala, S, Esposito, M, and Fuchs, A. An unusual variant of composite lymphoma: A short case report and review of the literature. Arch Pathol Lab Med (2000) 124:1376–8. doi:10.1043/0003-9985(2000)124<1376:AUVOCL>2.0.CO;2
5. Strickler, JG, Amsden, TW, and Kurtin, PJ. Small B-cell lymphoid neoplasms with coexisting T-cell lymphomas. Am J Clin Pathol (1992) 98:424–9. doi:10.1093/ajcp/98.4.424
6. Trimech, M, Letourneau, A, Missiaglia, E, De Prijck, B, Nagy-Hulliger, M, Somja, J, et al. Angioimmunoblastic T-Cell lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma: A novel form of composite lymphoma potentially mimicking richter syndrome. Am J Surg Pathol (2021) 45:773–86. doi:10.1097/pas.0000000000001646
7. Tomita, S, Kikuti, YY, Carreras, J, Sakai, R, Takata, K, Yoshino, T, et al. Monomorphic epitheliotropic intestinal T-Cell lymphoma in asia frequently shows SETD2 alterations. Cancers (Basel) (2020) 12:E3539. doi:10.3390/cancers12123539
8. Chen, C, Gong, Y, Yang, Y, Xia, Q, Rao, Q, Shao, Y, et al. Clinicopathological and molecular genomic features of monomorphic epitheliotropic intestinal T-Cell lymphoma in the chinese population: A Study of 20 cases. Diagn Pathol (2021) 16:114. doi:10.1186/s13000-021-01173-5
9. Kim, M, Lee, E, Zang, DY, Kim, HJ, Kim, HY, Han, B, et al. Novel genes exhibiting DNA methylation alterations in korean patients with chronic lymphocytic leukaemia: A methyl-CpG-binding domain sequencing study. Sci Rep (2020) 10:1085. doi:10.1038/s41598-020-57919-6
10. Diamantopoulos, PT, Sofotasiou, M, Georgoussi, Z, Giannakopoulou, N, Papadopoulou, V, Galanopoulos, A, et al. Prognostic significance of signal transducer and activator of transcription 5 and 5b expression in EPSTEIN-Barr virus-positive patients with chronic lymphocytic leukemia. Cancer Med (2016) 5:2240–8. doi:10.1002/cam4.804
11. Biran, A, Yin, S, Kretzmer, H, Ten Hacken, E, Parvin, S, Lucas, F, et al. Activation of notch and myc Signaling via B-cell-restricted depletion of Dnmt3a generates a consistent murine model of chronic lymphocytic leukemia. Cancer Res (2021) 81:6117–30. doi:10.1158/0008-5472.Can-21-1273
12. Mutzbauer, G, Maurus, K, Buszello, C, Pischimarov, J, Roth, S, Rosenwald, A, et al. SYK expression in monomorphic epitheliotropic intestinal T-Cell lymphoma. Mod Pathol (2018) 31:505–16. doi:10.1038/modpathol.2017.145
13. Quinquenel, A, Fornecker, LM, Letestu, R, Ysebaert, L, Fleury, C, Lazarian, G, et al. Prevalence of BTK and PLCG2 mutations in a real-life CLL cohort still on ibrutinib after 3 years: A FILO group study. Blood (2019) 134:641–4. doi:10.1182/blood.2019000854
14. Messina, M, Del Giudice, I, Khiabanian, H, Rossi, D, Chiaretti, S, Rasi, S, et al. Genetic lesions associated with chronic lymphocytic leukemia chemo-refractoriness. Blood (2014) 123:2378–88. doi:10.1182/blood-2013-10-534271
15. Parker, H, Rose-Zerilli, MJ, Larrayoz, M, Clifford, R, Edelmann, J, Blakemore, S, et al. Genomic disruption of the histone methyltransferase SETD2 in chronic lymphocytic leukaemia. Leukemia (2016) 30:2179–86. doi:10.1038/leu.2016.134
16. Roberti, A, Dobay, MP, Bisig, B, Vallois, D, Boéchat, C, Lanitis, E, et al. Type II enteropathy-associated T-cell lymphoma features a unique genomic profile with highly recurrent SETD2 alterations. Nat Commun (2016) 7:12602. doi:10.1038/ncomms12602
17. Moffitt, AB, Ondrejka, SL, McKinney, M, Rempel, RE, Goodlad, JR, Teh, CH, et al. Enteropathy-associated T cell lymphoma subtypes are characterized by loss of function of SETD2. J Exp Med (2017) 214:1371–86. doi:10.1084/jem.20160894
18. Tausch, E, Schneider, C, Robrecht, S, Zhang, C, Dolnik, A, Bloehdorn, J, et al. Prognostic and predictive impact of genetic markers in patients with CLL treated with obinutuzumab and venetoclax. Blood (2020) 135:2402–12. doi:10.1182/blood.2019004492
19. Kohlhaas, V, Blakemore, SJ, Al-Maarri, M, Nickel, N, Pal, M, Roth, A, et al. Active Akt signaling triggers CLL toward richter transformation via overactivation of Notch1. Blood (2021) 137:646–60. doi:10.1182/blood.2020005734
20. Kim, JA, Hwang, B, Park, SN, Huh, S, Im, K, Choi, S, et al. Genomic profile of chronic lymphocytic leukemia in Korea identified by targeted sequencing. PLoS One (2016) 11:e0167641. doi:10.1371/journal.pone.0167641
21. Riches, JC, Davies, JK, McClanahan, F, Fatah, R, Iqbal, S, Agrawal, S, et al. T cells from CLL patients exhibit features of T-Cell exhaustion but retain capacity for cytokine production. Blood (2013) 121:1612–21. doi:10.1182/blood-2012-09-457531
22. Goolsby, CL, Kuchnio, M, Finn, WG, and Peterson, L. Expansions of clonal and oligoclonal T cells in B-cell chronic lymphocytic leukemia are primarily restricted to the CD3(+)CD8(+) T-cell population. Cytometry (2000) 42:188–95. doi:10.1002/1097-0320(20000615)42:3<188:aid-cyto5>3.0.co;2-q
23. Martinez, A, Pittaluga, S, Villamor, N, Colomer, D, Rozman, M, Raffeld, M, et al. Clonal T-cell populations and increased risk for cytotoxic T-cell lymphomas in B-CLL patients: clinicopathologic observations and molecular analysis. Am J Surg Pathol (2004) 28:849–58. doi:10.1097/00000478-200407000-00002
24. Went, P, Agostinelli, C, Gallamini, A, Piccaluga, PP, Ascani, S, Sabattini, E, et al. Marker expression in peripheral T-cell lymphoma: A proposed clinical-pathologic prognostic score. J Clin Oncol (2006) 24:2472–9. doi:10.1200/jco.2005.03.6327
25. Kanavaros, P, Boulland, ML, Petit, B, Arnulf, B, and Gaulard, P. Expression of cytotoxic proteins in peripheral T-cell and natural killer-cell (NK) lymphomas: Association with extranodal site, NK or tgammadelta phenotype, anaplastic morphology and CD30 expression. Leuk Lymphoma (2000) 38:317–26. doi:10.3109/10428190009087022
Keywords: case report, chronic lymphocytic leukemia, composite lymphoma, small lymphocytic lymphoma, monomorphic epitheliotropic intestinal T-cell lymphoma
Citation: Zhang B, Zhang Y, Li Q, Jiang Q, Chu W, Gong H, Li R and Ji H (2022) Case report: Chronic lymphocytic leukemia/small lymphocytic lymphoma and monomorphic epitheliotropic intestinal T-cell lymphoma: A composite lymphoma. Pathol. Oncol. Res. 28:1610653. doi: 10.3389/pore.2022.1610653
Received: 14 June 2022; Accepted: 25 October 2022;
Published: 07 December 2022.
Edited by:
Agota Szepesi, Semmelweis University, HungaryCopyright © 2022 Zhang, Zhang, Li, Jiang, Chu, Gong, Li and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Ji, aGV6ZWppaG9uZ0BzaW5hLmNvbQ==
 Bing Zhang1
Bing Zhang1 Hong Ji
Hong Ji