Abstract
Anastomosing haemangioma (AH) is a newly described distinct vascular neoplasm that histologically may confuse with well-differentiated angiosarcoma (AS) for those who are unfamiliar with this rare entity. We aimed to identify molecular genetic differences between AHs and ASs by carrying out immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), and next-generation sequencing (NGS). Immunohistochemically, all six cases showed positivity for cyclinD1 and pERK. All cases of AH showed focal weak positive reaction for p53 and MIB-1, and the IHCs for HIF-1α were all negative for all three cases. Those three cases of angiosarcoma revealed strong, diffuse positivity for p53, 50%–70% MIB-1 labelling, and multifocal, moderate to strong HIF-1α expression. To further clarify the difference in p53 expression, we carried out a FISH which revealed 17p polysomy in all three ASs whereas copy number aberration was absent in the AH group. In one AH case, the GNA11 c.627G > T nucleotide variant was detected. Due to the rarity and overlapping morphological features, AH might be difficult to separate from other vascular tumours, in particular from well-differentiated AS also featured by mild hyperchromatic, hobnail-like endothelial cells. The potential molecular differences between these two entities presented here may be used in support of the correct diagnosis.
Introduction
Anastomosing haemangioma (AH) is a newly described distinct vascular neoplasm first reported in the genitourinary system. Later, distinct anatomical locations have also been reported including in the visceral organs [1–3] and paraspinal soft tissue [3]. Morphologically, AHs are characterized by loosely lobulated proliferations of capillary-like vessels with anastomosing, sinusoid patterns. They are lined by endothelial cells exhibiting hobnail-like appearances with mildly hyperchromatic nuclei protruding into the lumen, which is often confused with well-differentiated angiosarcoma (AS) for those who are unfamiliar with this rare entity. On the other hand, AHs also contain intravascular thrombi, intracytoplasmic eosinophilic hyaline globules and occasionally, extramedullary haematopoiesis, whereas multilayering nuclei, brisk mitotic activity and infiltrating into the adjacent tissue are usually absent. Recently, it has been reported that approximately 70% of AHs harbour activating GNAQ mutations, specifically at codon 209, providing molecular evidence of the neoplastic nature of AHs [4]. Furthermore, activating mutation GNA11 (codon 209), and GNA14 (codon 205), the paralogues of GNAQ have also been documented indicating the molecular heterogeneity of AHs [5, 6]. Mutations of GNAQ, GNA11 and GNA14 further trigger the MAPK signalling pathway which leads to uveal melanoma and congenital haemangioma formations. This article will investigate the potential molecular genetic differences between AHs and ASs by carrying out immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), and next-generation sequencing (NGS) molecular analysis from three AHs and three ASs.
Materials and Method
Histology and Immunohistochemistry
Haematoxylin and eosin (HE)-stained sections of all cases were reviewed by the same pathologist. After sectioning of 4 μm slides from formalin-fixed, paraffin-embedded blocks, deparaffinization in xylene and rehydration in a series of decreasing concentrations of ethanol were performed. Antigen retrieval using either the Bond Epitope Retrieval Solution 1 (pH∼6) or the Bond Epitope Retrieval Solution 2 (pH∼9) (Leica Microsystems, Wetzlar, Germany) was carried out at 99∼100°C for 20∼30 min. The slides were then treated with cyclinD1 (ready to use, clone SP4-R, Roche, Basel, Switzerland), p53 (1:700, clone Do-7, Dako, Agilent Technologies Company, Santa Clara, CA, United States), pERK (1:200, clone D13.14.4E, Cell Signaling Technology, Danvers, MA, United States), HIF-1α (1:400, polyclonal, GeneTex, Irvine, CA, United States) and MIB-1 (1:100, clone MIB-1, Dako, Agilent Technologies Company, Santa Clara, CA, United States) separately. Immunostaining was conducted on the Leica BOND-MAX™ autostainer (Leica Microsystems, Wetzlar, Germany) and the peroxidase/DAB Bond™ Polymer Refine Detection System (Leica Microsystems, Wetzlar, Germany) was utilized for visualization purposes.
All protocols have been approved by the author’s respective Institutional Review Board for human subjects (IRB reference number: IV/8465-3/2021/EKU).
Fluorescence In Situ Hybridization
Fluorescence in situ hybridization (FISH) was performed using XL TP53/17cen dual colour deletion probe to detect TP53 gene amplification/deletion on FFPE samples according to the manufacturer’s protocol (Metasystems, Altlussheim, Germany). The probe contains the chromosome 17 centromere for ploidy control. Denaturation and hybridization were performed in a hybridization chamber (StatSpin ThermoBrite, Abbott Laboratories, Abbott Park, IL, United States). Slides were denatured at 85°C for 5 min, and hybridization was subsequently performed overnight at 37°C. Fluorescence images were archived with the Isis imaging system (MetaSystems, Altlussheim, Germany).
Next-Generation Sequencing
QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) was used for FFPE tissue DNA extraction. The isolations were carried out according to the manufacturer’s instructions. The DNA concentrations were measured in the Qubit dsDNA HS Assay Kit using a Qubit 4.0 Fluorometer (Thermo Fisher Scientific, Waltham, MA, United States). For NGS analysis, libraries were constructed using AmpliSeq for Illumina Focus Panel (Illumina, San Diego, CA, United States) targeted resequencing assay for 52 genes with known relevance to solid tumours, and Accel-Amplicon Comprehensive TP53 Panel (Swift Biosciences, Integrated DNA Technologies, Coralville, IA, United States) for TP53 sequencing.
The indexed libraries were then presented to Illumina MiSeq System (MiSeq Reagent kit v3 600 cycles, Illumina, San Diego, CA, United States). The libraries pooling, denaturation and dilution were carried out according to the manufacturer’s protocol. The finished loading concentration was 8 pM libraries and 1% PhiX. Captured libraries were sequenced with a paired-end run to obtain 2 × 150 bp reads with at least 250X depth of coverage.
The fastq files were analyzed with the NextGENe software (version 2.4.2; SoftGenetics, State College, PA, United States) for identifying single-nucleotide variants (SNVs) as well as insertions and deletions (indels). For the alignment, the human reference genome GRCh37 (equivalent UCSC version hg19) was used. The sequence quality was evaluated and the cutoff was determined to be 5% variant allele frequency (VAF). Massive insertion/deletion (>50 bp) and compound structural changes can not be captured by this method. The results were determined using the latest version of the Human Genome Variation Society nomenclature.
Results
Clinicopathological Features
The six cases of AHs and ASs included in this study were diagnosed at our institute between 2018 and 2021. For the AH group, all three patients were female with an age bracket of 52–76 years old. Two cases presented with AH within the ovary, and one from the kidney. All three cases were well-circumscribed, and the size varied between 1.8 and 3.5 cm. All three cases received complete surgical excision. For the AS group, one male and two female patients were included with ages between 44 and 81 years old. The tumours arose from the chest wall, breast, and brain, respectively and the size ranged between 2.3 and 3.6 cm with irregular borders and incomplete excision. Further clinical information is illustrated in Table 1.
TABLE 1
| Diagnosis | ID | Gender | Age | Location | Size (cm) | Margin | Complete excision | MIB-1 (%) | CyclinD1 | pERK | p53 | CA-IX | HIF-1α | NGS Result | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anastomosing Haemangioma | 1 | F | 68 | Right ovary | 1.8 | Well circumscribed | Yes | 1 | Strong, diffuse | + | Weak, focal | — | — | Negative | Alive |
| 2 | F | 76 | Right ovary | 3.5 | Well circumscribed | Yes | 1 | Strong, diffuse | + | Negative | — | — | Negative | DOD (NET of the duodenum) | |
| 3 | F | 52 | Right kidney | 1.2 | Well circumscribed | Yes | 1 | Strong, diffuse | + | Weak, focal | — | — | GNA11 positive | Alive | |
| Well-differentiated Angiosarcoma | 4 | F | 68 | Right chest wall | 2.7 | Poorly circumscribed | No | 30 | Strong, diffuse | + | Strong, diffuse | — | N/C+ | Negative | DOD after 9 months |
| 5 | M | 44 | Brain | 3.6 | Poorly circumscribed | No | 20 | Strong, diffuse | + | Moderate, diffuse | — | N+ | Negative | DOD after 4.5 months | |
| 6 | F | 81 | Breast | 2.3 | Poorly circumscribed | No | 45 | Strong, diffuse | + | Moderate, diffuse | — | N+ | MAP2K1 and MYC positive | DOD after 13 months |
Clinicopathological information of anastomosing haemangioma and angiosarcoma cases (DOD, died of disease. NET, neuroendocrine tumour).
A good prognosis was recorded for the AH group, as two out of the three patients remain alive (based on follow-up data). The third patient, however, passed away due to complications from another tumour (grade 1 neuroendocrine tumour of the duodenum). A poor prognosis is seen in the AS group as all three cases died of disease within the follow-up period of 4.5 and 13 months after the initial pathological diagnosis.
Histological Features Including Immunohistochemistry
The histologic features of all AHs consisted of capillary-sized vascular channels in lobulated and solid patterns with rare mitoses. Hobnailing of endothelial cells with mild cytologic atypia was also noted (Figure 1 case 1–3). Vessels containing thrombi and intracytoplasmic eosinophilic globules were found in all three cases, whereas extramedullary haematopoiesis was absent. The stroma was fibrosclerotic with focal myxoid degeneration. Red blood cell extravasation, hemosiderin pigment and intertumoral adipose tissue can be found as well. The peripheral areas show chronic inflammatory cell infiltration. No noticeable necrosis was seen. The three angiosarcoma cases (Figure 1 case 4–6), on the contrary, showed bizarre nuclear atypia, infiltrative growth pattern with significant mitosis and tumour necrosis. Immunohistochemically, all six cases showed positivity for cyclinD1 and pERK. All cases of AH showed focal weakly positive for p53 and MIB-1 labelling index (less than 1%), and the IHCs for HIF-1α were all negative for all three cases. Those three cases of angiosarcoma revealed strong, diffuse positivity for p53, 50%–70% for MIB-1, and multifocal, moderate to strong HIF-1α expression (two cases intranuclear and one case intranuclear and cytoplasmic positivity).
FIGURE 1
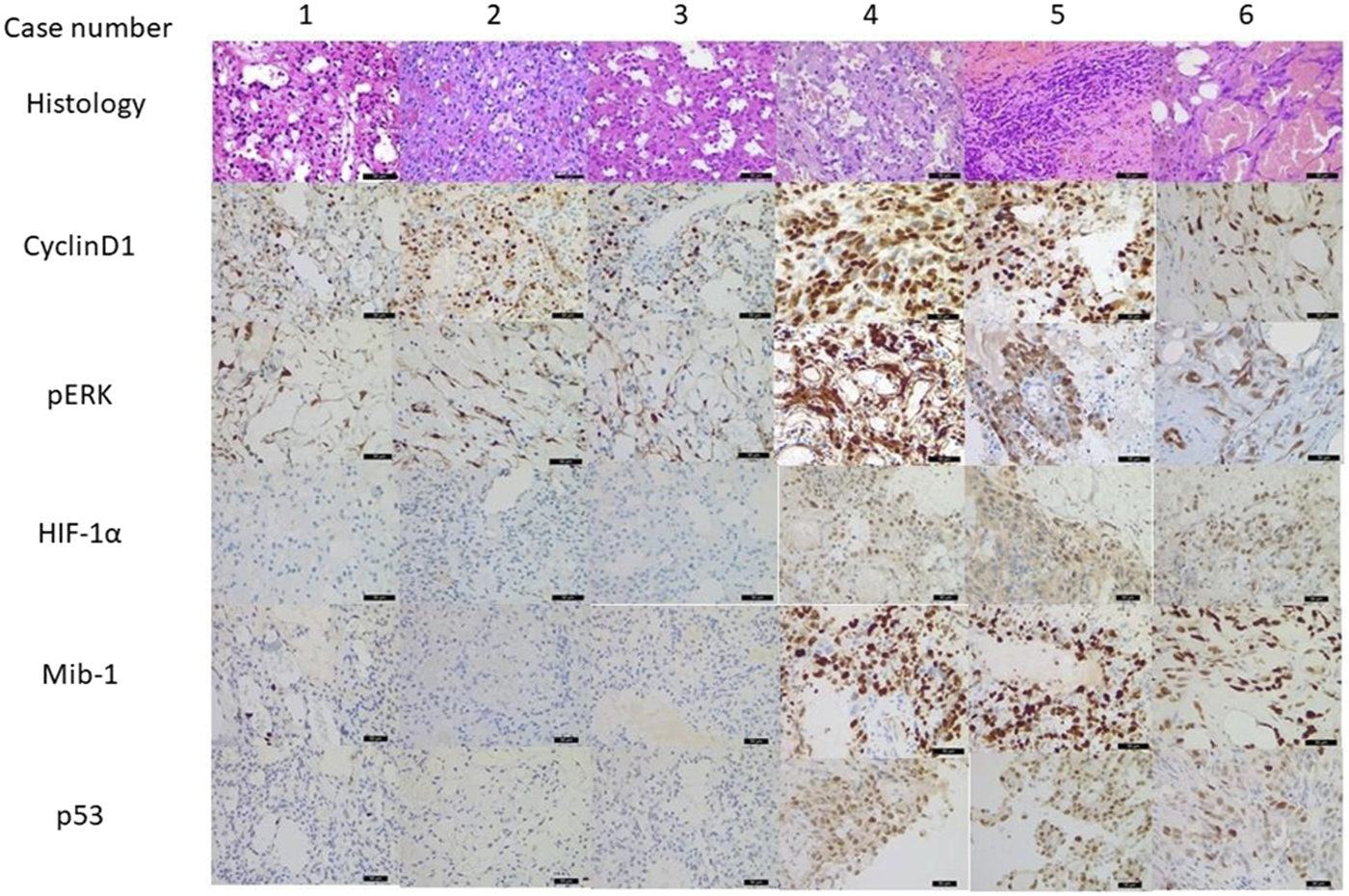
Morphology and immunohistochemistry result from anastomosing haemangiomas (AH, case 1–3) and angiosarcomas (AS, case 4–6) (×400 magnification). All six cases showed positivity for cyclinD1 and pERK indicating MAPK pathway activation. AHs also showed focal weakly positive for p53 and low MIB-1 positivity, and HIF-1α were negative for all three cases. ASs revealed strong, diffuse positivity for p53, significant higher MIB-1 labelling, and multifocal, moderate to strong HIF-1α expression.
Fluorescence In Situ Hybridization
To further clarify the difference in p53 expression between AHs and ASs, we carried out a FISH examination which revealed polysomy in all three ASs (Figure 2). Genetic aberration was absent in the AH group.
FIGURE 2
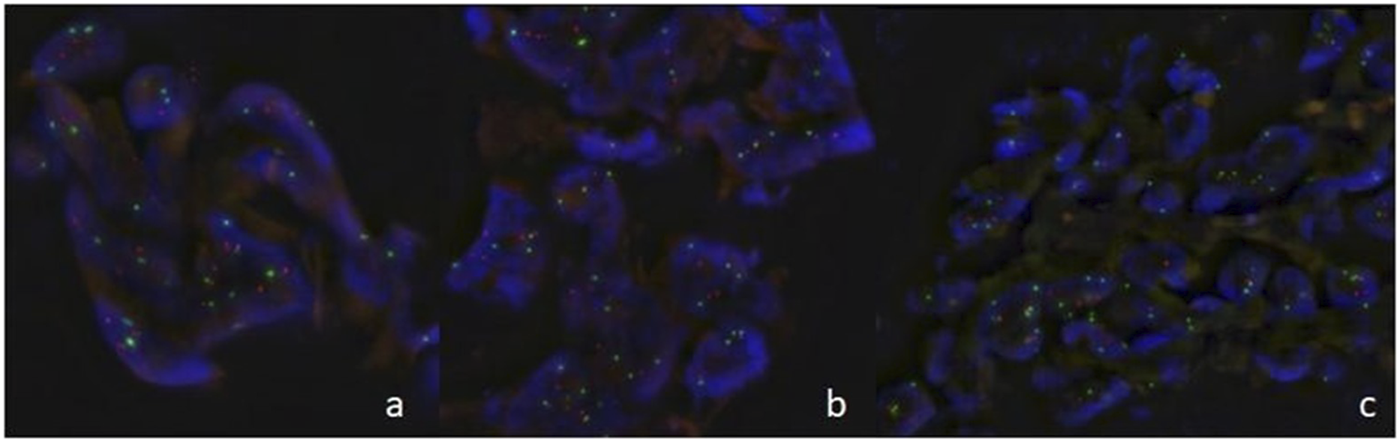
Fluorescence in situ hybridization in angiosarcomas ((A): case 4, (B): case 5, (C): case 6) showed of multiple dots for TP53 gene (orange) and chromosome 17 centromere (green) indicating TP53 gene polysomy.
NGS-Based Mutation Analysis
Using the AmpliSeq Focus Panel in one AH case the GNA11 c.627G > T; p. Q209H nucleotide variant was detected with 11% VAF. The other two AH cases were considered negative concerning this NGS panel.
When sequencing the AS samples, the molecular aberration was detected in only one case. MAP2K1 c.166C > A; p.Q56K and MYC c.217A > C; p.T73P alterations were observed in the positive AS sample with 37.97% and 22.3% VAF, respectively.
Because of differences between AH and AS cases p53 IHC staining, NGS was performed, which did not detect SNVs and small indels in the TP53 gene.
Discussion
Due to its rarity and overlapping morphological features, AH may be confused with other vascular tumours particularly well-differentiated AS due to the mild hyperchromatic, hobnail-like endothelial cells. Nevertheless, AHs usually possess differentiating factors such as the presence of lobulated contours and absence of prominent nuclear atypia, multilayering nuclei, high mitotic activity, infiltrating-dissecting growth pattern and massive tumour necrosis (Figure 3). In our study, the mitotic activity labelled by MIB-1 in our three AH cases was less than 1% compared ASs (>50%). Additionally, intravascular fibrin thrombi, peripheral adipose tissue, and, although not in our cases, extramedullary haematopoiesis can also serve as additional diagnostic clues for AHs. Furthermore, radiological information is crucial to separate AH from angiosarcoma as well [7].
FIGURE 3
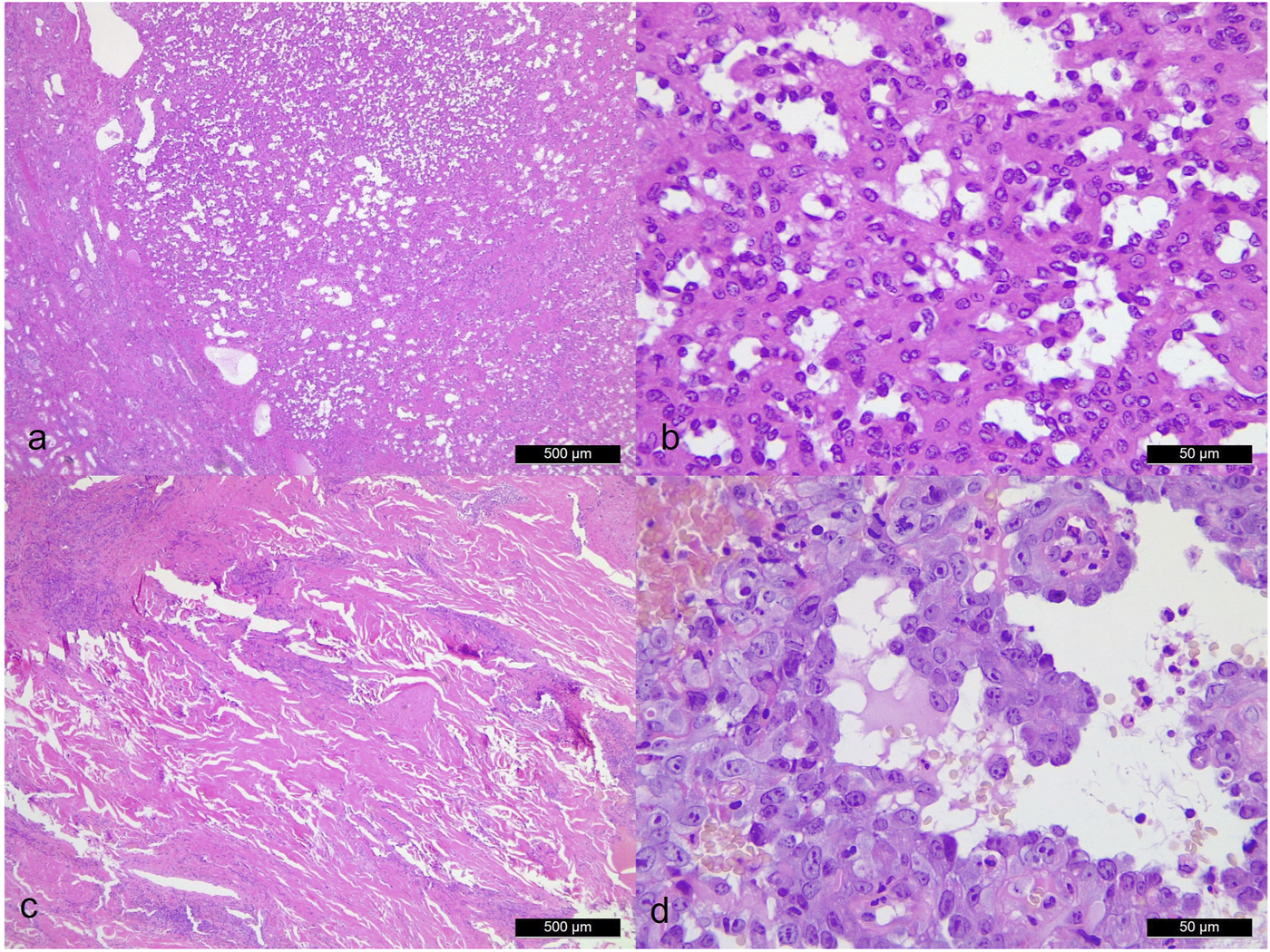
Morphological comparison between anastomosing hemangioma [case 3. (A): ×40 magnification. (B): ×400 magnification] and angiosarcoma [case 4. (C): ×40 magnification. (D): ×400 magnification]. Angiosarcoma shows significant cytologic atypia, endothelial cell multilayering and broadly infiltrative pattern.
GNAQ encodes G-protein subunit alpha q, together with its paralogues, GNA11, GNA14, and GNA15, comprise the alpha q subfamily of G proteins, which linked G-protein coupled receptors are the upstream components of ERK. It is known that mutations in GNAQ at codon 209, alter a region within the catalytic GTPase domain, resulting in constitutive activity which contributes to the oncogenesis of blue naevus and uveal melanoma [8]. Mutual exclusivity among GNAQ and its paralogue mutations have also been documented [9]. In our study, we identified one case of an AH harboured GNA11 mutation at codon 209, while the other two failed to present with hotspot mutations at neither GNAQ nor GNA11. Due to the restriction of our molecular study panel, which only assayed GNAQ and GNA11, we cannot exclude the possibility that two cases lacking mutations in GNAQ, and GNA11 might carry a mutation in the other genes within the signalling pathway. Intriguingly, a GNAQ/11 mutation is not entirely specific for AH as other types of vascular lesions such as congenital vascular malformation or cherry haemangioma [6, 10, 11] also carry the same genetic mutation. This implies the significance of clinical and morphological correlation to achieve the correct diagnosis.
The mitogen-activated protein kinase (MAPK) cascade is a highly conserved module involved in various cellular functions including cell proliferation, differentiation, and migration. Several proteins are involved in this pathway including extracellular signal-regulated kinases (ERK), which connect to G proteins through a multitude of distinct signal transduction pathways under dual phosphorylation on Tyr204 and Thr202 for ERK1 (p44), and Tyr187 and Thr185 for ERK2 (p42). Once activated, a signal is transmitted by phosphorylation of neighbouring proteins [12, 13]. The highly sensitive monoclonal antibody used in our study directly targets ERK1 (pThr202/pTyr204) and ERK2 (pThr185/pTyr187) and can serve as direct evidence of MAPK activation. In our study both AHs and ASs show both intranuclear and intracytoplasmic staining, together with intranuclear cyclinD1 positivity, implying the MAPK pathway is activated in both tumour groups, which is not surprising since it is crucial for angiogenesis [14]. However, we found the AS group revealed strong p53 expression, to further clarify this result, we carried out FISH which revealed p53 polysomy in all three ASs and a negative p53 result in the AH cases. Furthermore, we are interested in whether the microenvironment might play a role in these two entities by staining HIF-1α which shows two cases of multifocal intranuclear and one case of both intracytoplasmic and intranuclear positivity in angiosarcoma and negative for all AHs. Our data indicate that the MAPK pathway is triggered in both AH and AS by different genetic aberrations.
Apart from angiosarcomas, AH should also be differentiated from other vascular neoplasms as well such as retiform hemangioendothelioma, hobnail hemangioma, and sinusoidal hemangioma. They typically involve the skin and deep soft tissue or visceral occurrences are uncommon. Additionally, retiform hemangioendotheliomas show long, branching vascular channels lined by distinctly hyperchromatic, hobnailed endothelial cells with intraluminal papillations. Hobnail hemangiomas often have a distinctive clinical appearance and grow in a biphasic, wedge-shaped pattern, with dilated, superficial vessels lined by hobnail endothelial cells and a deeper dermal proliferation of capillaries forming slitlike spaces. Sinusoidal hemangiomas posses larger, gaping vessels with fibrotic walls and tend to lack adipocytic metaplasia, small thrombi, and extramedullary hematopoiesis, which is relatively common in AHs.
In conclusion, in our manuscript, we aimed to characterize general pathological features of AHs and compare them with a small selection of AS cases. While the three AH samples from the same institution represent a significant collection due to the rarity of the lesion, relatively more frequent ASs was included to highlight basic morphological and biological differences reflected by our setting. Regarding the specific molecular characteristics in AS our results were in close agreement with earlier publications, demonstrating the involvement of the TP53 gene [15]. We also demonstrated one case of AHs harbouring a GNA11 mutation which, besides conforming to its clonal nature, also serves as an important molecular signature to distinguish AHs from well-differentiated ASs. We addressed the potential molecular differences between these two entities. Nevertheless, due to the small sample size and its rarity, a larger scale study is needed to elucidate this issue. Clinical information and histology feature still serve as the gold standard for the correct diagnosis.
Statements
Data availability statement
The data presented in this study is available upon request from the corresponding author. The data is not publicly available due to the protective rights of the patients.
Ethics statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Hungarian Medical Research Council (protocol code IV/8465-3/2021/EKU). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Y-CC: data curation, formal analysis, investigation, project administration, and writing—original draft preparation; LB: methodology and formal analysis; GM: funding acquisition, investigation, resources, supervision, and writing—review and editing; and AM: conceptualization, investigation, methodology, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Montgomery E Epstein JI . Anastomosing Hemangioma of the Genitourinary Tract. Am J Surg Pathol (2009) 33:1364–9. 10.1097/PAS.0b013e3181ad30a7
2.
Lin J Bigge J Ulbright TM Montgomery E . Anastomosing Hemangioma of the Liver and Gastrointestinal Tract. Am J Surg Pathol (2013) 37:1761–5. 10.1097/PAS.0b013e3182967e6c
3.
John I Folpe AL . Anastomosing Hemangiomas Arising in Unusual Locations. Am J Surg Pathol (2016) 40:1084–9. 10.1097/PAS.0000000000000627
4.
Bean GR Joseph NM Gill RM Folpe AL Horvai AE Umetsu SE . Recurrent GNAQ Mutations in Anastomosing Hemangiomas. Mod Pathol (2017) 30:722–7. 10.1038/modpathol.2016.234
5.
Lim YH Bacchiocchi A Qiu J Straub R Bruckner A Bercovitch L et al GNA14 Somatic Mutation Causes Congenital and Sporadic Vascular Tumors by MAPK Activation. Am J Hum Genet (2016) 99:443–50. 10.1016/j.ajhg.2016.06.010
6.
Liau J-Y Lee J-C Tsai J-H Chen C-C Chung Y-C Wang Y-H . High Frequency of GNA14, GNAQ, and GNA11 Mutations in Cherry Hemangioma: a Histopathological and Molecular Study of 85 Cases Indicating GNA14 as the Most Commonly Mutated Gene in Vascular Neoplasms. Mod Pathol (2019) 32:1657–65. 10.1038/s41379-019-0284-y
7.
Cheon PM Rebello R Naqvi A Popovic S Bonert M Kapoor A . Anastomosing Hemangioma of the Kidney: Radiologic and Pathologic Distinctions of a Kidney Cancer Mimic. Curr Oncol (2018) 25:220–3. 10.3747/co.25.3927
8.
Van Raamsdonk CD Bezrookove V Green G Bauer J Gaugler L O’Brien JM et al Frequent Somatic Mutations of GNAQ in Uveal Melanoma and Blue Naevi. Nature (2009) 457:599–602. 10.1038/nature07586
9.
Urtatiz O Haage A Tanentzapf G Van Raamsdonk CD . Crosstalk with Keratinocytes Causes GNAQ Oncogene Specificity in Melanoma. Elife (2021) 10:e71825. 10.7554/eLife.71825
10.
Ayturk UM Couto JA Hann S Mulliken JB Williams KL Huang AY et al Somatic Activating Mutations in GNAQ and GNA11 Are Associated with Congenital Hemangioma. Am J Hum Genet (2016) 98:1271. 10.1016/j.ajhg.2016.05.010
11.
Couto JA Ayturk UM Konczyk DJ Goss JA Huang AY Hann S et al A Somatic GNA11 Mutation Is Associated with Extremity Capillary Malformation and Overgrowth. Angiogenesis (2017) 20:303–6. 10.1007/s10456-016-9538-1
12.
Flores K Yadav SS Katz AA Seger R . The Nuclear Translocation of Mitogen-Activated Protein Kinases: Molecular Mechanisms and Use as Novel Therapeutic Target. Neuroendocrinology (2019) 108:121–31. 10.1159/000494085
13.
Xu F Zhao L-J Liao T Li Z-C Wang L-L Lin P-Y et al Ononin Ameliorates Inflammation and Cartilage Degradation in Rat Chondrocytes with IL-1β-induced Osteoarthritis by Downregulating the MAPK and NF-κB Pathways. BMC Complement Med Ther (2022) 22:25. 10.1186/s12906-022-03504-5
14.
Tsai H-L Yeh Y-S Chang Y-T Yang I-P Lin C-H Kuo C-H et al Co-existence of Cyclin D1 and Vascular Endothelial Growth Factor Protein Expression Is a Poor Prognostic Factor for UICC Stage I-III Colorectal Cancer Patients after Curative Resection. J Surg Oncol (2013) 107:148–54. 10.1002/jso.23243
15.
Salter DMM Griffin M CulleyTeo JSJR Gomez-Cuadrado LMK Sims AHHL Henderson NCBVG . Development of Mouse Models of Angiosarcoma Driven by P53. Dis Model Mech (2019) 12:dmm038612. 10.1242/dmm.038612
Summary
Keywords
angiosarcoma, anastomosing haemangioma, immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), next-generation sequencing (NGS), GNA11 mutation
Citation
Chang Chien Y-C, Beke L, Méhes G and Mokánszki A (2022) Anastomosing Haemangioma: Report of Three Cases With Molecular and Immunohistochemical Studies and Comparison With Well-Differentiated Angiosarcoma. Pathol. Oncol. Res. 28:1610498. doi: 10.3389/pore.2022.1610498
Received
07 April 2022
Accepted
23 June 2022
Published
01 August 2022
Volume
28 - 2022
Edited by
Andrea Ladányi, National Institute of Oncology (NIO), Hungary
Updates
Copyright
© 2022 Chang Chien, Beke, Méhes and Mokánszki.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Che Chang Chien, dr.changchien.yiche@med.unideb.hu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.