- Department of General Surgery Two, Shanxi Province Cancer Hospital, Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences, Cancer Hospital Affiliated to Shanxi Medical University, Taiyuan, China
Aim: In this study, we aimed to evaluate the associations of vascular endothelial growth factor (VEGF) gene single nucleotide polymorphisms (SNPs) and its interaction with current smoking with gastric cancer (GC) risk in the Chinese Han population.
Methods: We used logistic regression model to test the association between VEGF gene polymorphism and the risk of GC. The association strength was evaluated by odds ratio (OR) and 95% confidence interval (CI) calculated using logistic regression. Generalized multifactor dimensionality reduction (GMDR) was used to analyze the effect of the interaction between VEGF gene and current smoking on GC risk.
Results: Logistic regression analysis showed that the risk of GC was significantly higher in rs10434 -G allele carriers than that in AA genotype carriers (AG + GG and AA), and the adjusted OR (95% CI) = 1.64 (1.24–2.08). In addition, we found a significantly higher GC risk in subjects with rs833061-T allele than those with CC allele (CT + TT and CC), adjusted or (95% CI) = 1.43 (1.10–1.87). We also found a statistically significant two- locus model (p = 0.018), including rs10434 and current smoking, indicating a significant interaction between rs10434 and current smoking on the risk of GC. Hierarchical analysis found that current smokers with AG or GG genotype have the highest GC risk, compared to never- smokers with AA genotype, OR (95% CI) = 2.43 (1.64–3.28).
Conclusion: We found that rs10434 -G and rs833061-T alleles, gene- environment interaction between rs10434, and current smoking were all related to increased GC risk.
Introduction
Gastric cancer (GC) is a common cancer type globally. Newly diagnosed gastric cancer account for 5.7% of all cancers, with over a million cases a year. The high incidence rate and high mortality rate make GC one of the most important cancers threatening human health [1, 2]. GC is the third leading cause of cancer-related death. The incidence rate differs depending on country, and has a higher incidence in East Asia, Eastern Europe, and South America. But the incidence rate is relatively low in Oceania, Africa, and North America [3]. Previous studies have reported some risk factors for GC, the most common of which include Helicobacter pylori infection, tobacco smoking, low fiber intake, high salt or smoked food intake, and genetic factors [4].
Vascular endothelial growth factor (VEGF) is an effective mitogen in the endothelium. Its main role is to regulate angiogenesis and postnatal vascular remodeling. Its expression is up- regulated under various pathophysiological conditions [5]. Therefore, vascular endothelial growth factor is a lymphangiogenic growth factor, which plays an important role in tumor lymphangiogenesis by activating VEGF receptor [6]. VEGF gene is located on chromosome 6p21.1 and contains 9 exons [7]. Previously, several studies have reported the relationship between VEGF gene single nucleotide polymorphism (SNP) and several cancer’s risk, including bladder cancer [8], lung cancer [9], and renal cell carcinoma [10]. However, the etiology of the relationship between VEGF gene SNPs and GC risk is very complicated and yet to be completely understood. In addition, the pathogenesis of GC involves not only genetic factors but also environmental factors; no study has focused on the synergistic effect between VEGF gene and environmental factors. Therefore, the main purpose of this study is to evaluate whether there is a statistical association between VEGF gene polymorphism and GC risk in the Chinese Han population, and the impact of the interaction between VEGF gene and environmental lifestyle factors on GC risk.
Methods
Study Population
All participants in this study were recruited from Shanxi cancer hospital from June 2016 to July 2020. Those with malignant diseases other than gastric cancer, such as cardiovascular disease and other tumors, were not included in the case group. The control group was randomly selected from healthy individuals of a chronic non-communicable disease screening program in the area. The control group was finally selected by matching the subjects of the case group by 2:1 according to age and gender. Consequently, a total of 1,451 eligible participants were included in the study; the mean age of all participants were 64.0 ± 10.1 years. A total of 483 eligible gastric cancer patients were included in the case group and 968 healthy participants were included in the control group. We designed a simple questionnaire to collect participants’ general demographic information and clinical and biochemical index data. Whole blood samples were collected from each participant. Written informed consent was obtained from all participants.
Genotyping Methods
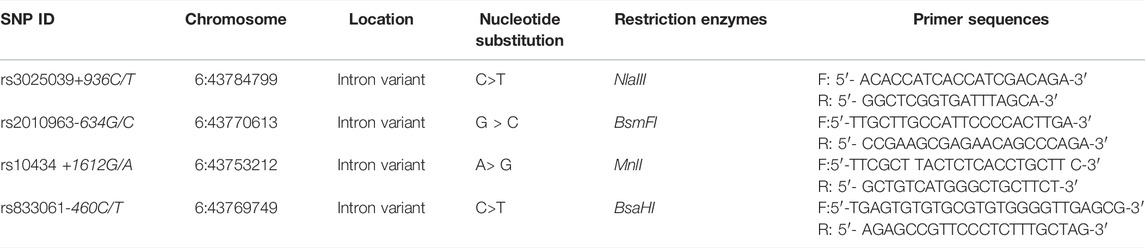
A total of four SNPs within VEGF gene were selected, including rs10434, rs3025039, rs2010963, and rs833061. We collected 3 ml blood samples from all participants, and these blood samples were treated with EDTA and stored in a −20°C refrigerator. The subject’s DNA was extracted according to the instructions of the DNA blood micro- Kit (Qiagen, Hilden, Germany). In this study, genotypes of four selected SNPs were determined by using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay as previously described [11, 12]. The product was digested with restriction enzymes, which were shown in Table 1, and analyzed onto 2% agarose gel. All staff involved in genotype testing were blinded to the basic information and phenotypes of all participants and, after the experiment, we repeated the test on a randomly selected 10% of the samples to ensure the accuracy and reproducibility of the test results. Repeat test results are 100% consistent with the first test.
Statistical Analysis
All data were tested using SPSS 22.2 software, and we calculated the mean and standard deviation (SD) for continuous variables conforming to normal distribution and calculated the percentage for classified variables. The χ2 testing was used for comparison of categorical variables, and the T-test was used to compare the mean and SD. The online software (SNPStats: https://www.snpstats.net/) was used for analysis on association between four SNPs and GC risk. Generalized multifactor dimensionality reduction (GMDR) [13] was employed to test the interaction between the four SNPs and current smoking. The consistency of cross validation and the accuracy of test balance and symbolic test were calculated to evaluate the interaction of each selection.
Results
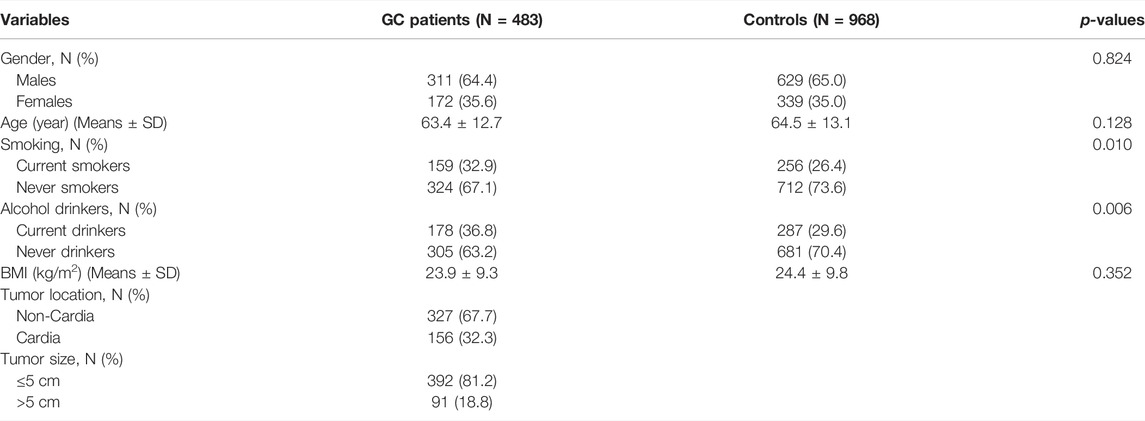
A total of 1,451 participants with an average age of 64.0 ± 10.1 years were selected, made up of 483 GC patients and 968 healthy controls. Table 2 shows the general demographic characteristics and clinical indicators in GC patients and controls. The mean age, BMI, and percentage of males were not significantly different between GC patients and controls. The percentage for current smoking and alcohol drinking were significantly higher in GC patients than in controls. In addition, 67.7% of the GC patients were non-cardia and the tumor size of 81.2% GC patients was ≤5 cm.
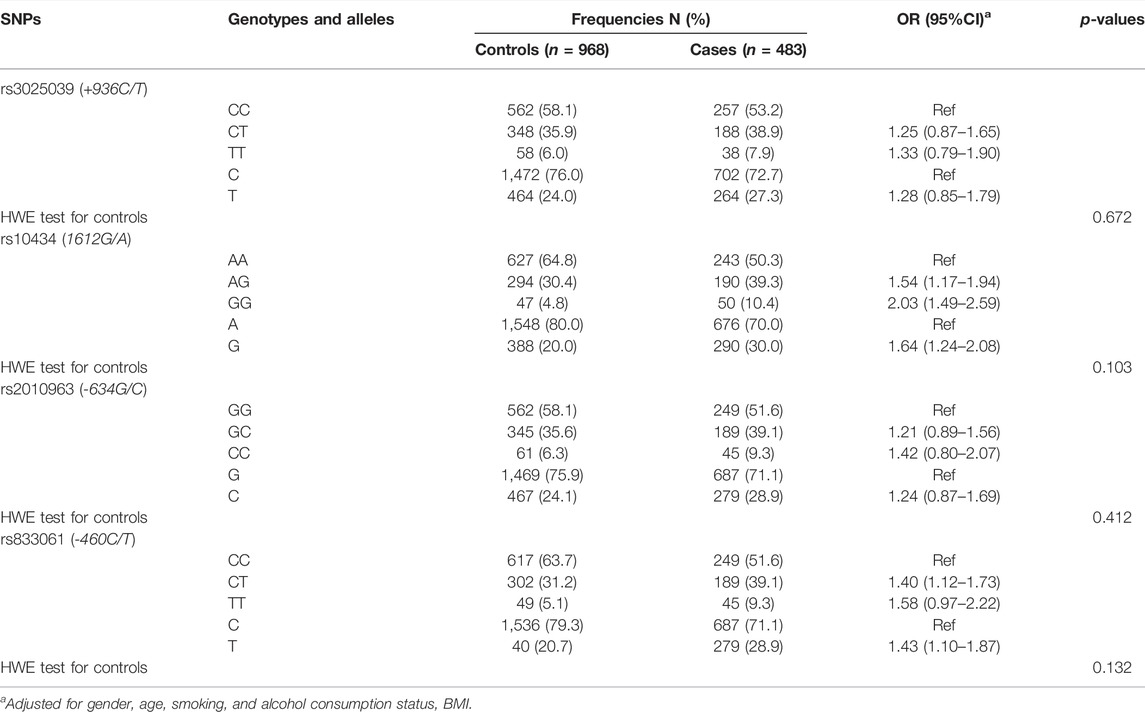
The genotype distribution in the control group was consistent with the HWE (All p- values more than 0.05). The allele frequencies of rs10434 -G and rs833061-T were significantly higher in GC patients than that in control group (30.0% and 20.0%, 28.9% and 20.7%, respectively). Logistic regression analysis showed that the risk of GC was significantly higher in rs10434 -G allele carriers than that in AA genotype carriers (AG + GG and AA), and the adjusted OR (95% CI) = 1.64 (1.24–2.08). In addition, we found a significantly higher GC risk in subjects with rs833061-T allele than those with CC allele (CT + TT and CC), adjusted or (95% CI) = 1.43 (1.10–1.87) (Table 3).
The GMDR model was used to evaluate the synergy effect between four VEGF gene SNPs and current smoking on the susceptibility to GC (Table 4). We found a statistically significant two- locus model (p = 0.018), including rs10434 and current smoking, indicating a significant interaction between rs10434 and current smoking on the risk of GC. The cross- validation consistency of the two- locus model was 10/10, the test accuracy was 0.632, and p- value was 0.018. To obtain the odds ratios and 95%CI for the joint effects of rs10434 polymorphism and current smoking on GC susceptibility, we conducted hierarchical analysis for interaction between rs10434 and current smoking on GC risk by using logistic regression. We found that current smokers with AG or GG genotype have the highest GC risk, compared to never- smokers with AA genotype, OR (95%CI) = 2.43 (1.64–3.28), after adjustment for gender, age, alcohol consumption status, and BMI (Table 5).
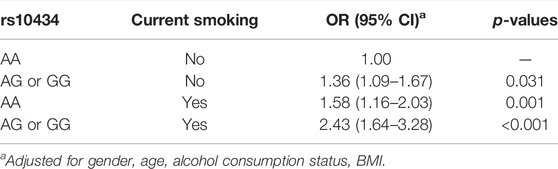
TABLE 5. Hierarchical analysis for interaction between rs10434 and current smoking on GC risk by using logistic regression.
Discussion
In our study, we investigated the relationship of four SNPs of VEGF gene with GC risk. The results indicated that the risk of GC was significantly higher in rs10434 -G allele carriers than that in AA genotype carriers. In addition, we also found a significantly higher GC risk in subjects with rs833061-T allele than those with CC allele. Nevertheless, after several covariates’ adjustment, we found that no statistically significant correlation existed between rs3025039, rs2010963, and GC risk. Previously, several studies [14–19] focused on the association between SNPs of VEGF gene and GC risk in different populations. According to the research of Zhou et al [14] in the Chinese Han population, VEGF rs10434 (+ 1612 G/A) gene polymorphism may be related to a higher risk of gastric cancer, and the difference of genotype distribution may be related to Lauren classification and the location of gastric cancer, but they also found that rs2010963 and rs833061 were not associated with GC risk. In a Japanese population, Tahara et al [15] suggested that the G1612A mutation in VEGF gene was statistically associated with the risk of GC, but the association between C936T polymorphism and GC susceptibility was not statistically significant. A meta-analysis by Hong et al [16] including these studies showed that the 1612a allele of VEGF gene is a recessive susceptibility site of gastric cancer, with a 60% increased risk. However, the meta-analysis results of Zhuang et al [17] showed that people carrying VEGF -634 G allele may increase the risk of gastric cancer, while VEGF +1612 G/A G allele may reduce the risk of gastric cancer. No association was found between + 936C/T and - 460C/T polymorphisms and susceptibility to gastric cancer. Liu et al. [18] conducted meta-analysis on Asian, European, and American populations respectively, and found that in the overall population, VEGF-634 G> C GG genotype was associated with the reduction of gastric cancer risk, VEGF- 634 G> C- C allele and GG genotype were associated with the risk in Caucasian people, while in the Asian population, VEGF + 1612G/A locus was significantly associated with the risk of GC. In our study, we also verified a significant relationship of rs10434 and rs833061 with GC susceptibility in the Chinese population. The mechanisms underlying the association between the VEGF gene and gastric cancer are not exhaustive. Previous studies have suggested that VEGF is one of the most important growth factors in the process of lymphatic angiogenesis and can play an important role in the progression of lymphangiogenesis in several tumors by activating the VEGF receptor. In the stomach, enhanced VEGF gene expression has been identified as contributing to the healing of GI lesions [20, 21]. In addition, gastric adenocarcinomas frequently show high levels of VEGF expression accompanied by an increase in intratumoral microvessel density. Higher levels of VEGF expression can also be observed in pre-cancerous gastric lesions, such as precancerous lesions, chronic atrophic gastritis, and intestinal chemosis [22].
GC susceptibility was influenced by many risk factors, including environmental and genetic factors, and the synergistic effect between gene and lifestyle factors [23]. Previously, the association between smoking habit and GC risk has been reported [24–27], and to date, no study focused on the effect of VEGF gene- smoking interaction on GC risk. In the current study, GMDR software was employed to investigate the interaction among four SNPs of VEGF gene and current smoking. We found a significant interaction between rs10434 and current smoking on the risk of GC; current smokers with AG or GG genotype have the highest GC risk, compared to never- smokers with AA genotype. The biological mechanism of the interaction between VEGF gene and smoking on the risk of gastric cancer is not very clear. Previous studies [27, 28] have confirmed that VEGF gene and smoking are related to the inflammatory response in the human body. Perhaps there is the same biological mechanism between them, resulting in a significant change to susceptibility of gastric cancer when they exist at the same time.
Some limitations existed in this study and should be explained. Firstly, there are too many SNPs in VEGF gene. In this study, only four SNPs are selected. These four SNPs could not represent all VEGF genes, but these SNPs are often mentioned in previous studies, and the results are different. Secondly, in this study, all environmental factors listed in Table 2 were included in GMDR software, but just the significant results were listed in Table 4; in the future, more lifestyle factors should be included in the interaction analysis. Lastly, we did not collect information about Helicobacter pylori infection, so the bias due to H. pylori infection may exist in this study.
In conclusion, our research shows rs10434 -G and rs833061-T alleles, gene- environment interaction between rs10434, and current smoking were all related to increased GC risk. In the future, more functional experiments are needed to verify the results obtained in our study.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by the Shanxi Province Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LW: Study concepts, study design, manuscript preparation, manuscript editing, and manuscript review. ZG: Definition of intellectual content. SX: Literature research, data acquisition, and data analysis. YZ: Experimental studies and statistical analysis. All authors have read and approved the manuscript.
Acknowledgments
The writing of this paper was supported by the Shanxi Province Cancer Hospital. Our gratitude is extended to the leadership of the hospital, the help of colleagues, and the cooperation of the included subjects.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bray, F, Ferlay, J, Soerjomataram, I, Siegel, RL, Torre, LA, and Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi:10.3322/caac.21492
2. Rawla, P, and Barsouk, A. Epidemiology of Gastric Cancer: Global Trends, Risk Factors and Prevention. Prz Gastroenterol (2019) 14(1):26–38. doi:10.5114/pg.2018.80001
3. Karimi, P, Islami, F, Anandasabapathy, S, Freedman, ND, and Kamangar, F. Gastric Cancer: Descriptive Epidemiology, Risk Factors, Screening, and Prevention. Cancer Epidemiol Biomarkers Prev (2014) 23:700–13. doi:10.1158/1055-9965.EPI-13-1057
4. Alipour, M. Molecular Mechanism of Helicobacter Pylori-Induced Gastric Cancer. J Gastrointest Cancer (2021) 52(1):23–30. doi:10.1007/s12029-020-00518-5
5. Alagappan, VK, Willems-Widyastuti, A, Seynhaeve, AL, Garrelds, IM, ten Hagen, TL, Saxena, PR, et al. Vasoactive Peptides Upregulate mRNA Expression and Secretion of Vascular Endothelial Growth Factor in Human Airway Smooth Muscle Cells. Cell Biochem Biophys (2007) 47(1):109–18. doi:10.1385/cbb:47:1:109
6. Eroglu, A, Ersoz, C, Karasoy, D, and Sak, S. Vascular Endothelial Growth Factor (VEGF)-C, VEGF-D, VEGFR-3 and D2-40 Expressions in Primary Breast Cancer: Association with Lymph Node Metastasis. Adv Clin Exp Med (2017) 26(2):245–9. doi:10.17219/acem/58784
7. Vincenti, V, Cassano, C, Rocchi, M, and Persico, G. Assignment of the Vascular Endothelial Growth Factor Gene to Human Chromosome 6p21.3. Circulation (1996) 93:1493–5. doi:10.1161/01.cir.93.8.1493
8. Fu, D, Li, P, Cheng, W, Tian, F, Xu, X, Yi, X, et al. Impact of Vascular Endothelial Growth Factor Gene-Gene and Gene-Smoking Interaction and Haplotype Combination on Bladder Cancer Risk in Chinese Population. Oncotarget (2017) 8(14):22927–35. doi:10.18632/oncotarget.15287
9. Naykoo, NA, Dil-Afroze, , , Rasool, R, Shah, S, Ahangar, AG, Bhat, IA, et al. Single Nucleotide Polymorphisms, Haplotype Association and Tumour Expression of the Vascular Endothelial Growth Factor (VEGF) Gene with Lung Carcinoma. Gene (2017) 608:95–102. doi:10.1016/j.gene.2017.01.007
10. Ratnasari, N, Nurdjanah, S, Sadewa, AH, and Hakimi, M. The Role of Vascular Endothelial Growth Factor -634 G/C and its Soluble Receptor on Chronic Liver Disease and Hepatocellular Carcinoma. Arab J Gastroenterol (2016) 17(2):61–6. doi:10.1016/j.ajg.2016.06.005
11. Rezaei, M, Hashemi, M, Sanaei, S, Mashhadi, MA, and Taheri, M. Association between Vascular Endothelial Growth Factor Gene Polymorphisms with Breast Cancer Risk in an Iranian Population. Breast Cancer (Auckl) (2016) 10:85–91. doi:10.4137/BCBCR.S39649
12. Tie, Z, Bai, R, Zhai, Z, Zhang, G, Zhang, H, Zhao, Z, et al. Single Nucleotide Polymorphisms in VEGF Gene are Associated with an Increased Risk of Osteosarcoma. Int J Clin Exp Pathol (2014) 7(11):8143–9.
13. Xu, HM, Xu, LF, Hou, TT, Luo, LF, Chen, GB, Sun, XW, et al. GMDR: Versatile Software for Detecting Gene-Gene and Gene-Environment Interactions Underlying Complex Traits. Curr Genomics (2016) 17(5):396–402. doi:10.2174/1389202917666160513102612
14. Zhou, Y, Li, N, Zhuang, W, and Wu, X. Vascular Endothelial Growth Factor (VEGF) Gene Polymorphisms and Gastric Cancer Risk in a Chinese Han Population. Mol Carcinog (2011) 50(3):184–8. doi:10.1002/mc.20703
15. Tahara, T, Shibata, T, Nakamura, M, Yamashita, H, Yoshioka, D, Hirata, I, et al. Effect of Polymorphisms in the 3’ Untranslated Region (3’-UTR) of Vascular Endothelial Growth Factor Gene on Gastric Cancer and Peptic Ulcer Diseases in Japan. Mol Carcinog (2009) 48(11):1030–7. doi:10.1002/mc.20554
16. Hong, TT, Cai, DY, Wu, XH, and Hua, D. Three Vascular Endothelial Growth Factor Polymorphisms (−460C>T, −2578C>a, 1612G>A) with Cancer Risk: A Meta-Analysis Based on 30 Case-Control Studies. Cancer Investig (2011) 29(7):472–7. doi:10.3109/07357907.2011.597811
17. Zhuang, M, Peng, Z, Wang, J, and Su, X. Vascular Endothelial Growth Factor Gene Polymorphisms and Gastric Cancer Risk: A Meta-Analysis. J BUON (2017) 22(3):714–24.
18. Liu, W, Dong, Z, Hu, R, and Wang, C. Association of Vascular Endothelial Growth Factor (VEGF) Gene Polymorphisms with Gastric Cancer and its Development, Prognosis, and Survival. Technol Cancer Res Treat (2018) 17:1533034617753810. doi:10.1177/1533034617753810
19. Zhou, LP, Luan, H, Dong, XH, Jin, GJ, Man, DL, and Shang, H. Vascular Endothelial Growth Factor +936C/T Polymorphism and Gastric Cancer Risk: A Meta-Analysis. Exp Ther Med (2011) 2(5):931–6. doi:10.3892/etm.2011.278
20. Takahashi, Y, Cleary, KR, Mai, M, Kitadai, Y, Bucana, CD, and Ellis, LM. Significance of Vessel Count and Vascular Endothelial Growth Factor and its Receptor (KDR) in Intestinal-type Gastric Cancer. Clin Cancer Res (1996) 2:1679–84.
21. Maeda, K, Kang, SM, Onoda, N, Ogawa, M, Kato, Y, Sawada, T, et al. Vascular Endothelial Growth Factor Expression in Preoperative Biopsy Specimens Correlates with Disease Recurrence in Patients with Early Gastric Carcinoma. Cancer (1999) 86:566–71. doi:10.1002/(sici)1097-0142(19990815)86:4<566::aid-cncr4>3.0.co;2-1
22. Feng, CW, Wang, LD, Jiao, LH, Liu, B, Zheng, S, and Xie, XJ. Expression of P53, Inducible Nitric Oxide Synthase and Vascular Endothelial Growth Factor in Gastric Precancerous and Cancerous Lesions: Correlation with Clinical Features. BMC Cancer (2000) 29:8. doi:10.1186/1471-2407-2-8
23. Lao, X, Peng, Q, Lu, Y, Li, S, Qin, X, Chen, Z, et al. Glutathione S-Transferase Gene GSTM1, Gene-Gene Interaction, and Gastric Cancer Susceptibility: Evidence from an Updated Meta-Analysis. Cancer Cel Int (2014) 14(1):127. doi:10.1186/s12935-014-0127-3
24. Praud, D, Rota, M, Pelucchi, C, Bertuccio, P, Rosso, T, Galeone, C, et al. Cigarette Smoking and Gastric Cancer in the Stomach Cancer Pooling (StoP) Project. Eur J Cancer Prev (2018) 27(2):124–33. doi:10.1097/CEJ.0000000000000290
25. Kim, SA, Choi, BY, Song, KS, Park, CH, Eun, CS, Han, DS, et al. Prediagnostic Smoking and Alcohol Drinking and Gastric Cancer Survival: A Korean Prospective Cohort Study. Korean J Gastroenterol (2019) 73(3):141–51. doi:10.4166/kjg.2019.73.3.141
26. Ferro, A, Morais, S, Rota, M, Pelucchi, C, Bertuccio, P, Bonzi, R, et al. Tobacco Smoking and Gastric Cancer: Meta-Analyses of Published Data versus Pooled Analyses of Individual Participant Data (StoP Project). Eur J Cancer Prev (2018) 27(3):197–204. doi:10.1097/CEJ.0000000000000401
27. Ugur, MG, Kutlu, R, and Kilinc, I. The Effects of Smoking on Vascular Endothelial Growth Factor and Inflammation Markers: A Case-Control Study. Clin Respir J (2018) 12(5):1912–8. doi:10.1111/crj.12755
28. Rovina, N, Papapetropoulos, A, Kollintza, A, Michailidou, M, Simoes, DC, Roussos, C, et al. Vascular Endothelial Growth Factor: an Angiogenic Factor Reflecting Airway Inflammation in Healthy Smokers and in Patients with Bronchitis Type of Chronic Obstructive Pulmonary Disease? Respir Res (2007) 8(1):53. doi:10.1186/1465-9921-8-53
Keywords: gastric cancer, smoking, interaction, single nucleotide polymorphisms, vascular endothelial growth factor
Citation: Wang L, Xiao S, Zheng Y and Gao Z (2022) Interaction Between Vascular Endothelial Growth Factor Gene Polymorphism and Smoking on Gastric Cancer Risk in Chinese Han Population. Pathol. Oncol. Res. 28:1610495. doi: 10.3389/pore.2022.1610495
Received: 07 April 2022; Accepted: 26 July 2022;
Published: 25 August 2022.
Edited by:
Francesca Sanguedolce, University of Foggia, ItalyCopyright © 2022 Wang, Xiao, Zheng and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Longyue Wang, d2FuZ2xvbmd5dWU2NjFAMTYzLmNvbQ==
 Longyue Wang
Longyue Wang Shuaishuai Xiao
Shuaishuai Xiao Yiming Zheng
Yiming Zheng