- 1Faculty of Medicine, Poznan University of Medical Sciences, Poznan, Poland
- 2Department of Pediatric Oncology, Hematology and Transplantology, Institute of Pediatrics, Poznan University of Medical Sciences, Poznan, Poland
Brentuximab vedotin is a conjugate drug used mainly in Hodgkin lymphoma, systemic and primary cutaneous anaplastic large cell lymphomas, and CD30-expressing peripheral T-cell lymphoma. We report a unique case of acute pancreatitis associated with brentuximab vedotin in a 17-year-old male patient suffering from classical Hodgkin lymphoma. Diagnosed in 2020, the patient was classified to an intermediate therapeutic group and disease’s grade was IIIAE. The patient was treated with brentuximab vedotin and bendamustine in the third line. Two weeks after the drug administration, the patient developed acute epigastric pain. Laboratory and radiological findings confirmed the clinical suspicion of acute pancreatitis that was managed with opioid pain medications, meropenem, parenteral nutrition, ondansetron and omeprazole. This is the first case report of brentuximab vedotin-associated acute pancreatitis in the pediatric patient reported in the literature to the best of our knowledge.
Introduction
Hodgkin lymphoma (HL) is one of the most common neoplasms among adolescents [1]. Thanks to advances in its treatment, a 5-year event-free survival rate exceeds 90% [2]. Adverse outcomes in the remaining 10% of patients are related to various factors, including advanced stage of disease, bulky disease, pleural and cardiac effusion [3–6]. In addition, male sex and initially slow response to commencing cycles of chemotherapy are adverse prognostic factors [3, 7]. Global results of HL treatment are excellent, yet refractory HL remains a therapeutic challenge. Due to the small percentage of complex cases, a limited number of clinical trials focus on refractory Hodgkin disease [2]. Moreover, another aspect that should be considered is the questionable ethics of clinical trials conducted among pediatric patients. Some of the agents may have different pharmacokinetics and side effects that may even affect a child’s development, hence reluctance to conduct such trials [8].
In the pediatric population, the leading protocol used for the first-line treatment of Hodgkin lymphoma was established by the European Network-Paediatric Hodgkin Lymphoma Study Group (EuroNet-PHL) [2]. This trial’s strategy focuses on the restriction of radiation therapy by including patients with Deauville scores 4 and 5 only and augmentation of chemotherapy intensity in intermediate and advanced HL [2]. Second-line and further lines of treatment comprise various schemes of chemotherapy, which may be combined with brentuximab vedotin (BV), nivolumab, or followed by autologous hematopoietic cell transplantation [2].
Brentuximab vedotin is an anti-CD30 directed antibody-drug conjugate to auristatin, initially used in adult patients with HL and anaplastic large cell lymphoma, refractory and relapsed [9, 10]. Now, its use is investigated among the pediatric population in several clinical trials [11, 12]. The pivotal study conducted by Locatelli et al. showed BV is an agent able to reduce late toxicity and adverse events among pediatric patients that stems from the administration of traditional chemotherapy [12]. In addition, it proved to help lead to autologous stem cell transplantation in these patients. The most common adverse reactions include peripheral neuropathy and neutropenia [11, 12]. While acute pancreatitis has been described in a few case series and case reports, all previously described patients were adults [13–15]. Brentuximab vedotin is the first agent that should be considered while planning the treatment of R/R HL with any novel agents [12]. The combination of brentuximab vedotin and bendamustine was introduced after studies conducted by Vinti et al. and McMillan et al. showed promising data for pediatric patients with little data on severe toxicity [16, 17].
In this case report, we describe a 17-year-old male patient with refractory Hodgkin lymphoma that was administered brentuximab vedotin and bendamustine (B) in his 4th cycle of treatment. He developed acute pancreatitis 2 weeks after the first infusion of BV. We also review other BV-associated pancreatitis case reports described in the literature, elucidate the possible mechanism of this adverse event and present the consensus on the treatment of AP in pediatric oncological patients.
Case Report
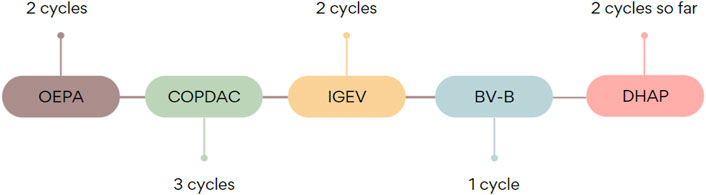
A currently 17-year-old overweight male patient (1.79 m, 90 kg, BMI 28.09) with a bulky (>200 ml) mediastinal tumor was diagnosed with nodular sclerosis classical HL, IIIAE grade, intermediate therapeutic group (TL-2) at the age of 16. Due to life-threatening pressure of the mass and superior vena cava syndrome, as well as venous thrombosis, the debulking operation was performed. At first, steroid treatment was implemented. Later, the patient was enrolled in the EuroNET-PHL-C2 clinical trial [18]. The patient was administered OEPA (vincristine sulfate 1.5 mg/m2, etoposide phosphate 125 mg/m2, doxorubicin hydrochloride 40 mg/m2, prednisone 60 mg/m2) chemotherapy. After two cycles, his group was changed to advanced (TL-3) due to mass penetration into the spinal canal in the thoracic Th2/Th3 segment, and he was qualified to COPDAC (cyclophosphamide 600 mg/m2, vincristine sulfate 1.4 mg/m2, dacarbazine 250 mg/m2, prednisone 40 mg/m2). Despite intensified chemotherapy regimen, an interim PET-CT scan showed progression (Deauville 5). Therefore, treatment was changed to IGEV (ifosfamide 2000 mg/m2, gemcitabine 800 mg/m2, vinorelbine 20 mg/m2, prednisolone 100 mg/m2). Later, brentuximab vedotin and bendamustine were introduced: 188.1 mg (90 mg/m2) bendamustine was administered twice and there was one infusion of 162 mg (1.8 mg/kg) brentuximab vedotin.
After 2 weeks from the first brentuximab vedotin administration, the patient presented to the emergency department with abdominal and back pain and vomiting. Physical examination revealed tachycardia, abdominal bloatedness, and disseminated pain in palpation of the whole abdomen. In laboratory tests inflammatory reaction was observed (CRP 5.32 mg%, leucocytes 17 990/mm3, neutrophils 15 870/mm3) and levels of pancreatic enzymes were increased (amylase 1665 U/L, lipase 3853 U/L). Triglycerides levels were within reference values—118 mg/dl and rose up to maximum of 170 mg/dl on the 10th day of hospitalization. Ultrasound investigations revealed a slightly enlarged pancreas as compared to age (head 1.9 cm, body 2.6 cm, tail 2.4 cm), peripancreatic adipose tissue of increased echogenicity and sign of free fluid in the peritoneal cavity. CT scan confirmed the inflammatory reaction of tissues surrounding the pancreas. The patient was administered pain medications - metamizole and tramadol, fluid therapy, meropenem, ondansetron and omeprazole. A gastric tube was also used. Due to the increasing severity of pain, its treatment was changed to morphine and nalbuphine. Parenteral feeding was introduced. During the following days, there was a significant increase in inflammatory parameters (maximum C-reactive protein 42 mg%), and therefore, the antibiotic therapy was intensified. Gradual clinical improvement of the patient and normalization of pancreatic enzymes was observed. However, the patient complained of strengthening symptoms resulting from the underlying Hodgkin lymphoma. The increase of inflammatory markers was also observed. Chest CT confirmed the progression of the disease and the rise in tumors’ infiltration. Oral steroid therapy was introduced and DHAP (cytarabine 2000 mg/m2, cisplatin 100 mg/m2, dexamethasone 40 mg/m2) chemotherapy regimen was planned. It was followed without any severe side-effects, except transient increase of transaminase level. The patient is still being treated with a DHAP regimen with a non-satisfactory result. It is planned he will be given nivolumab as the last-chance treatment and followed by autologous hematopoietic stem cell transplantation. Illustration of chemotherapy sequence is shown in Figure 1.
Discussion
Despite an excellent overall prognosis, refractory HL still poses a therapeutic challenge. The optimal therapy in R/R Hodgkin disease in pediatric patients has not been established due to the scarcity of data and lack of randomized trials that could compare possible approaches and their effectiveness. It is unknown whether standard-dose chemotherapy is better than high dose chemotherapy followed by autologous stem cell transplant; only small cohort national trials show that high dose chemotherapy is usually not needed [2]. EuroNet Pediatric Hodgkin Lymphoma group has thus issued guidelines on approaching refractory HL considering new possible therapies that include brentuximab vedotin. The panel also considers the stratification of patients into three subgroups depending on the time of relapse and previous treatment. The described patient qualifies to the high-risk group that includes patients that failed to achieve remission in PET-CT after two lines of salvage standard-dose therapy. Such patients are candidates for treatment with conventional high dose chemotherapy, autologous stem cell transplantation and additional therapies, i.e., biological drugs or experimental strategies.
The use of BV-B (brentuximab vedotin and bendamustine) has proved to have a non-dangerous safety profile. The use of these agents provokes a long-lasting response to the treatment and is a bridge to autologous stem cell transplantation in the future, providing the possibility of stem cell mobilization [2].
It is worth mentioning that the patient is overweight (BMI 28.09) and the dose of brentuximab vedotin is weight-dependent. The required dosage of BV for patients from 12 years old weighing between 50 and 100 kg is 1.8 mg/kg every 3 weeks or 1.2 mg/kg every 2 weeks. However, in younger children [19], treatment results were worse using these doses. Suri et al. elucidated in their pharmacokinetics analysis that the recommended dose suitable for all the patients (up to 100 kg) is 71.5 mg/m2 every 3 weeks and 47.7 mg/m2 every 2 weeks and it provides the same concentrations of the drug (AUC, area under curve). In the case of our patient, the calculated dose would therefore be 148.7 mg, which is a slightly smaller dose than the one calculated based on the patient’s weight. Nevertheless, statistical correlation between the dose of BV and acute pancreatitis or different adverse events has not been proven in literature yet. In the case of our patient, it is essential to keep in mind that high body mass index and adiposity increase the risk of AP and being overweight is a negative prognostic factor of AP severity [20–23].
Literature Review
Other BV-Induced Pancreatitis Cases Described in the Literature
Almost one-fifth of the BV patients present abdominal pain, which raises the question of whether BV-induced pancreatitis is underdiagnosed or truly rare. So far, only a few cases of brentuximab vedotin induced pancreatitis in patients with HL have been reported in the literature, of which two were fatal, but none of them occurred in the pediatric population. One fatal pancreatitis was reported at the beginning of the clinical trial (NCT01476410) [24], which led to the exclusion of patients with a history of AP. Then one patient without such history developed this toxicity. Urru et al. reported the first known case of BV-induced pancreatitis in a Hodgkin lymphoma patient [14]. Even though the patient developed symptoms of acute pancreatitis a few days after the second infusion of the drug, which differs, symptoms and clinical outcome were similar to our case and following case reports. Gandhi et al. describe a case series of 8 patients with acute pancreatitis that emerged in a median time of 26 days after the first exposure to brentuximab vedotin and 12 days after its recent administration [15]. Maradana et al. reported severe hypertriglyceridemia-induced acute pancreatitis 2 weeks after the first brentuximab treatment in a patient with T-cell skin lymphoma [13]. As mentioned earlier, BV is also useful in the treatment of CD30+ ALCL [12]. Chen et al. describe the first case of fatal pancreatitis on the 13th day in a patient suffering from ALCL [25]. All the reported cases of BV-induced pancreatitis are listed in Table 1. Interestingly, there is a case of polatuzumab-vedotin-piiq-induced pancreatitis reported in literature. No previous pancreas-related adverse events were reported during clinical trials with this agent [26].
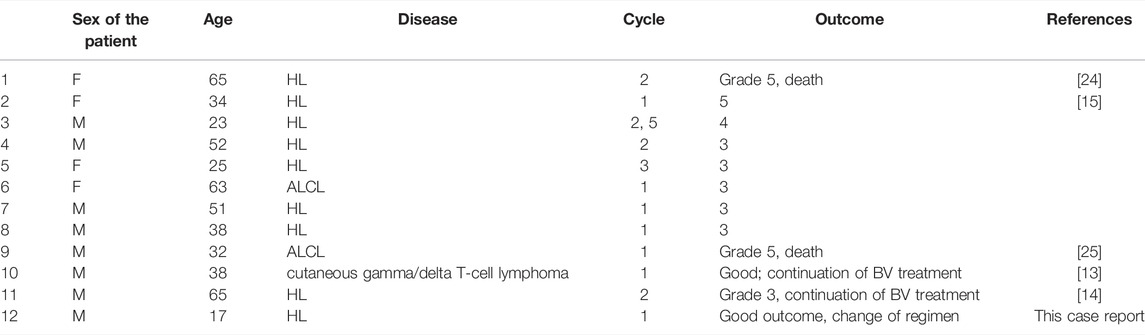
TABLE 1. The summary of all reported cases of pancreatitis associated with brentuximab vedotin treatment.
The vast majority of BV-induced AP cases are revealed after the first and second administration of the agent. In our case, the patient developed acute pancreatitis after 2 weeks from first and only brentuximab vedotin intake, which is consistent with previous reports. Three patients continued BV intake, 2 of which did without any adverse events and one of the patients developed pancreatitis again after three other infusions. This data is too scarce to say whether the administration of BV should be discontinued in all the cases or can be resumed with a benefit for the patient. For now, its readministration should be discouraged. Our patient had no chance to profit from this treatment as one cycle of BV proves too little to objectively assess its impact.
Patomechanism of BV-Induced Pancreatitis
The cause of BV-induced pancreatitis is still uncertain. Nevertheless, another case report suggests targeting variable low-level CD30 in the pancreas due to higher expression of in patients than in mouse immunoglobulin controls [15]. Also, other considered mechanisms of this adverse effect are circulating unconjugated monomethylauristatin E or the occurrence of isolated CD30-positive malignant cells in the pancreas [15]. Host-dependent reasons are also considered, such as predisposing genetic variants, which the best described are PRSS1, CTRC, SPINK1, CFTR [27].
Acute Pancreatitis as a Side Effect of Drugs Used in Pediatric Oncology
Acute pancreatitis may be caused by various drugs, also those used in pediatric oncology. However, diagnosis of drug-induced AP is often unclear and based only on clinical suspicion rather than evidence. In addition, many case reports do not exclude more common reasons for AP, such as gallstones, alcohol, hypertriglyceridemia, which makes it a real challenge to find the actual frequency of drug-associated pancreatitis [28].
In pediatric oncology, steroids, antibiotics such as tetracyclines and trimethoprim/sulfamethoxazole, asparaginase and mercaptopurine are associated with drug-induced AP [29]. The most widely known drug to cause AP is asparaginase, with the first case reported in the 1970s and one of the most important reasons to stop this drug [29, 30]. The evolution of asparaginase treatment and its new generations was crucial in improving the treatment results [31]. Approximately 2–18% of patients administered with asparaginase are affected by that side reaction [31]. Pathophysiology is yet unknown but is probably related to the mechanism of action of this drug. Asparaginase depletes external sources of asparagine, which is detrimental for lymphoblasts and affects organs with high protein expenditure like the pancreas [30]. The risk of AP is independent from dose or route of administration [30]. Another critical factor is the concomitant use of steroids, especially dexamethasone [32].
Possibly important risk factors are age, concurrent therapy with other anticancer drugs, some genetic variants and hypertriglyceridemia [30]. Asparaginase therapy may be reintroduced only when amylase or lipase is mildly elevated and the patient presents no clinical symptoms [30]. Nevertheless, more than 60% of re-exposed patients may have a relapse [33]. Compared to brentuximab vedotin, clinical symptoms, diagnostics methods and general outcomes are similar to those in generally healthy children [29]. However, immunodeficiency should be kept in mind and the adverse effects of other oncological drugs.
Bortezomib is another drug used in reinduction therapy of ALL in children that have been described to provoke acute pancreatitis [34]. Its mechanism of action is based on a reversible inhibition of proteasome subunit 26s, thereby inhibiting its chymotrypsin activity. However, this side effect is rare, occurring in 0,1–2% of cases [35]. The mechanism of inflammation is still unknown, but direct drug toxicity or allergic and immunological causes are considered [35]. Elevation of pancreatic enzymes is observed chiefly a few days after drug administration. It is possible to continue bortezomib therapy [35].
AP should also be considered an adverse effect of mercaptopurine; however, its frequency depends on the treated disease. For example, in inflammatory bowel disease patients, mercaptopurine-associated AP is a well-described complication of treatment, occurring in 2–6% of cases [36]. When it comes to hematological diseases, fewer reports can be found. Nevertheless, there are described cases in acute lymphoblastic leukemia [37].
Also, cytarabine may induce AP. Very few papers report that side effect in acute myeloblastic leukemia [38]. Therefore, the mechanism is not found, but previous exposure to asparaginase is considered.
AP may also be concerning in the stem cell transplantation scenario as it may be caused by tacrolimus or cyclosporine [39]. Very few case reports can be found with tacrolimus-associated AP, most of them after solid organ transplantation [40]. The majority of them reported high or very high concentrations of tacrolimus and cyclosporine was implemented instead [40].
Treatment of Pancreatitis in Pediatric Oncological Patients
Management of AP in leukemia/lymphoma patients should be more intensive than in less complicated cases. Due to the poorer oncological outcome after canceling the drug, it is imperative to assure whether there is pancreatitis, its severity and therefore a need to stop the treatment. In an asymptomatic course, when only enzymes are elevated (chemical pancreatitis), it is possible to continue drug administration [41].
Pharmacotherapy of AP remains a controversial topic and the use of antibiotics, protease inhibitors and octreotide are widely discussed. Prophylactic use of antibiotics is generally discouraged until infected necrosis is proved [42]. However, even in the case of acute necrotizing pancreatitis, the data is inconsistent. Two controlled, double‐blind studies and one meta-analysis show no benefit of antibiotics therapy, while two separate meta‐analyses concluded that the patients with proven pancreatic necrosis should receive either imipenem or meropenem prophylaxis [29, 43, 44, 45].
On the other hand, when l-asparaginase induced AP is concerned, authors suggest using broad-spectrum antibiotics, especially imipenem or meropenem [46]. This approach is seen in adult ALL patients as well, with recognition of no clear evidence that antibiotics prevent the development of sepsis or other complications [47].
All authors agree that supportive care is crucial [29]. Fluid therapy, proper nutrition and pain management are undoubtedly critical. Patient monitoring (cardiovascular, pulmonary and renal parameters) is essential to determine AP complications quickly.
Other Rare Complications and Toxicities Associated With Brentuximab Vedotin
BV may cause many more or less serious side effects, apart from AP, such as peripheral neuropathy, nausea, fatigue, neutropenia, diarrhea, pyrexia, vomiting, arthralgia, pruritus, myalgia [48].
The most fatal and rare toxicity is progressive multifocal leukoencephalopathy (PML) caused by JC infection [49]. To the best of our knowledge, nine cases have been reported so far and at least six of them are lethal [49]. PML is often a fatal disease caused by the reactivation of JCV. Currently, it is associated with HIV infection, yet less known is the fact that before the spread of HIV, patients with lymphoproliferative disorders were ones who mostly suffered from PML [49]. The disease is associated with chemotherapy, immunosuppressive medications as well as monoclonal antibodies, including natalizumab, rituximab and brentuximab vedotin [49]. The most common symptoms of PML are motor weakness, gait abnormality, sensory loss, speech disorders and visual abnormalities [50]. Product monograph of brentuximab vedotin accentuates the importance of symptom monitoring and proper diagnostics as soon as they emerge [51]. Drugs should be stopped in PML patients and are contraindicated for patients who have had PML [51].
Conclusion
It should be kept in mind that any epigastric pain in a patient that intakes brentuximab vedotin should be closely diagnosed as it may be a symptom of acute pancreatitis. While this drug is characterized by an overall safe profile and proves to be very efficient in treating refractory Hodgkin disease, acute pancreatitis is a severe adverse reaction, which requires immediate, adequate treatment and may lead to further complications. The direct link between BV and acute pancreatitis cannot be proved. Nonetheless, based on previous case reports, we wish to highlight such possibility and report such association in the pediatric patient.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
AW and KD, conceptualization; ET, MA, and CS, manuscript writing; ET, MA, CS, AW, and KD, final manuscript editing and review. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Miller, KD, Fidler-Benaoudia, M, Keegan, TH, Hipp, HS, Jemal, A, Siegel, RL, et al. Cancer Statistics for Adolescents and Young Adults, 2020. CA Cancer J Clin (2020) 443:6–59. doi:10.3322/caac.21637
2. Daw, S, Hasenclever, D, Mascarin, M, Fernández-Teijeiro, A, Balwierz, W, Beishuizen, A, et al. Risk and Response Adapted Treatment Guidelines for Managing First Relapsed and Refractory Classical Hodgkin Lymphoma in Children and Young People. Recommendations from the EuroNet Pediatric Hodgkin Lymphoma Group. HemaSphere (2020) 4:e329. doi:10.1097/HS9.0000000000000329
3. Smith, RS, Chen, Q, Hudson, MM, Link, MP, Kun, M, Weinstein, H, et al. Prognostic Factors for Children with Hodgkin's Disease Treated with Combined-Modality Therapy. J Clin Oncol (2003) 21:2026–33. doi:10.1200/JCO.2003.07.124
4. Schwartz, CL, Chen, L, McCarten, K, Wolden, S, Constine, LS, Hutchison, RE, et al. Childhood Hodgkin International Prognostic Score (CHIPS) Predicts Event-free Survival in Hodgkin Lymphoma: A Report from the Children's Oncology Group. Pediatr Blood Cancer (2017) 64(4):e26278. doi:10.1002/pbc.2627810.1002/pbc.26278
5. McCarten, KM, Metzger, ML, Drachtman, RA, Pei, Q, Friedman, DL, Schwartz, CL, et al. Significance of Pleural Effusion at Diagnosis in Pediatric Hodgkin Lymphoma: A Report from Children's Oncology Group Protocol AHOD0031. Pediatr Radiol (2018) 48(12):1736–44. doi:10.1007/s00247-018-4197-6
6. Marks, LJ, McCarten, KM, Pei, Q, Friedman, DL, Schwartz, CL, Kelly, KM, et al. Pericardial Effusion in Hodgkin Lymphoma: A Report from the Children’s Oncology Group AHOD0031 Protocol. Blood (2018) 132(11):1208–11. doi:10.1182/blood-2018-02-834465
7. Hutchings, M, Loft, A, Hansen, M, Pedersen, LM, Buhl, T, Jurlander, J, et al. FDG-PET after Two Cycles of Chemotherapy Predicts Treatment Failure and Progression-free Survival in Hodgkin Lymphoma. Blood (2006) 107(1):52–9. doi:10.1182/blood-2005-06-2252
8. Joseph, PD, Craig, JC, and Caldwell, PH. Clinical Trials in Children. Br J Clin Pharmacol (2015) 79:357–69. doi:10.1111/bcp.12305
9. Bazarbachi, A, Boumendil, A, Finel, H, Mohty, M, Castagna, L, Blaise, D, et al. Brentuximab Vedotin for Recurrent Hodgkin Lymphoma after Allogeneic Hematopoietic Stem Cell Transplantation: A Report from the EBMT Lymphoma Working Party. Cancer (2019) 125:90–8. doi:10.1002/cncr.31755
10. O'Connor, OA, Lue, JK, Sawas, A, Amengual, JE, Deng, C, Kalac, M, et al. Brentuximab Vedotin Plus Bendamustine in Relapsed or Refractory Hodgkin's Lymphoma: an International, Multicentre, Single-Arm, Phase 1-2 trialErratum in. Lancet Oncol (2018) 1919(23):257e137–66. doi:10.1016/S1470-2045(17)30912-9
11. Cole, PD, McCarten, KM, Pei, Q, Spira, M, Metzger, ML, Drachtman, RA, et al. Brentuximab Vedotin with Gemcitabine for Paediatric and Young Adult Patients with Relapsed or Refractory Hodgkin's Lymphoma (AHOD1221): A Children's Oncology Group, Multicentre Single-Arm, Phase 1–2 Trial. Lancet Oncol (2018) 19(9):1229–38. doi:10.1016/S1470-2045(18)30426-1
12. Locatelli, F, Mauz-Koerholz, C, Neville, K, Llort, A, Beishuizen, A, Daw, S, et al. Brentuximab Vedotin for Paediatric Relapsed or Refractory Hodgkin's Lymphoma and Anaplastic Large-Cell Lymphoma: a Multicentre, Open-Label, Phase 1/2 Study. Lancet Haematol (2018) 5:e450–61. doi:10.1016/S2352-3026(18)30153-4
13. Maradana, S, Akella, P, Nalluru, SS, Jindal, V, and Siddiqui, AD. Hypertriglyceridemia Induced Pancreatitis Due to Brentuximab Therapy: First Case Report. Cureus (2019) 11(7):e5138. doi:10.7759/cureus.5138
14. Urru, SAM, Mariotti, E, Carta, P, Massidda, S, Marcias, M, Murru, R, et al. Acute Pancreatitis Following Brentuximab Vedotin Therapy for Refractory Hodgkin Lymphoma: A Case Report. Drugs R D (2014) 14:9–11. doi:10.1007/s40268-014-0036-x
15. Gandhi, MD, Evens, AM, Fenske, TS, Hamlin, P, Coiffier, B, Engert, A, et al. Pancreatitis in Patients Treated with Brentuximab Vedotin: A Previously Unrecognized Serious Adverse Event. Blood (2014) 123(18):2895–7. doi:10.1182/blood-2014-03-561878
16. Vinti, L, Locatelli, F, Merli, P, Parasole, R, Buffardi, S, Pillon, M, et al. Brentuximab Vedotin in Combination with Bendamustine in Relapsed or Refractory Hodgkin Lymphoma: A Retrospective Analysis on 23 Paediatric Patients or Young Adults. Blood (2017) 130:1. doi:10.1182/blood.V130.Suppl_1.4090.4090
17. McMillan, A, O'Neil, AT, Townsend, W, Lambert, J, Virchi, A, Shah, R, et al. The Addition of Bendamustine to Brentuximab Vedotin Leads to Improved Rates of Complete Metabolic Remission in Children, Adolescents and Young Adults with Relapsed and Refractory Classical Hodgkin Lymphoma: A Retrospective Single-centre Series. Br J Haematol (2021) 192:e84–e87. doi:10.1111/bjh.17274
18. Körholz, D, Wallace, WH, and Landman-Parker, J. Euro-net-Paediatric Hodgkin’s Lymphoma Group (Euro-Net-PHL-C2): Second International Inter-group Study for Classical Hodgkin Lymphoma in Children and Adolescents (2015). Available at: https://clinicaltrials.gov/ct2/show/NCT02684708 (Accessed February 20, 2022).
19. Suri, A, Mould, DR, Song, G, Kinley, J, and Venkatakrishnan, K. Population Pharmacokinetics of Brentuximab Vedotin in Adult and Pediatric Patients with Relapsed/Refractory Hematologic Malignancies: Model-Informed Hypothesis Generation for Pediatric Dosing Regimens. J Clin Pharmacol (2020) 60(12):1585–97. doi:10.1002/jcph.1682
20. Pang, Y, Kartsonaki, C, Turnbull, I, Guo, Y, Yang, L, Bian, Z, et al. Metabolic and Lifestyle Risk Factors for Acute Pancreatitis in Chinese Adults: A Prospective Cohort Study of 0.5 Million People. Plos Med (2018) 15(8):e1002618. doi:10.1371/journal.pmed.1002618
21. Aune, D, Mahamat-Saleh, Y, Norat, T, and Riboli, E. High Body Mass Index and Central Adiposity Is Associated with Increased Risk of Acute Pancreatitis: A Meta-Analysis. Dig Dis Sci (2021) 66(4):1249–67. doi:10.1007/s10620-020-06275-6
22. Li, X, Guo, X, Ji, H, Niu, J, and Gao, P. Relationships between Metabolic Comorbidities and Occurrence, Severity, and Outcomes in Patients with Acute Pancreatitis: A Narrative Review. Biomed Res Int (2019) 2019:2645926. doi:10.1155/2019/2645926
23. Wang, SQ, Shu-jun, L, Quan-xin, F, Xiang-ying, F, Xu, L, Zhao, QC, et al. Overweight Is an Additional Prognostic Factor in Acute Pancreatitis: A Meta-Analysis. Pancreatology (2011) 11(2):92–8. doi:10.1159/000327688
24. Evens, AM, Advani, RH, Helenowski, IB, Fanale, M, Smith, SM, Jovanovic, BD, et al. Multicenter Phase II Study of Sequential Brentuximab Vedotin and Doxorubicin, Vinblastine, and Dacarbazine Chemotherapy for Older Patients with Untreated Classical Hodgkin Lymphoma. J Clin Oncol (2018) 36(30):3015–22. doi:10.1200/JCO.2018.79.0139
25. Ching Chen, C, and Su-Peng, Y. Fatal Pancreatitis Occurred in a Patient with Refractory CD30+ Anaplastic Large Cell Lymphoma after Brentuximab Vedotin Treatment. J Cancer Res Pract (2017) 4(1):35–7. doi:10.1016/j.jcrpr.2016.09.002
26. Anderson, K, Shehata, M, Singh, D, and Al-Ourani, M. Acute Pancreatitis Induced by Polatuzumab-Vedotin-Piiq in Combination with Bendamustine and Rituximab for Diffuse Large B-Cell Lymphoma. Cureus (2020) 12(9):e10299. doi:10.7759/cureus.10299
27. Whitcomb, DC. Genetic Risk Factors for Pancreatic Disorders. Gastroenterology (2013) 144(6):1292–302. doi:10.1053/j.gastro.2013.01.069
28. Tenner, S. Drug-Induced Acute Pancreatitis: Underdiagnosis and Overdiagnosis. Dig Dis Sci (2010) 55:2706–8. doi:10.1007/s10620-010-1367-2
29. Stefanović, M, Jazbec, J, Lindgren, F, Bulajić, M, and Löhr, M. Acute Pancreatitis as a Complication of Childhood Cancer Treatment.. Cancer Med (2016) 5(5):827–36. doi:10.1002/cam4.649
30. Raja, RA, Schmiegelow, K, and Frandsen, TL. Asparaginase-associated Pancreatitis in Children. Br J Haematol (2012) 159:18–27. doi:10.1111/bjh.12016
31. Hijiya, N, and van der Sluis, IM. Asparaginase-associated Toxicity in Children with Acute Lymphoblastic Leukemia. Leuk Lymphoma (2016) 57(4):748–57. doi:10.3109/10428194.2015.1101098
32. Teuffel, O, Kuster, SP, Hunger, SP, Conter, V, Hitzler, J, Ethier, MC, et al. Dexamethasone versus Prednisone for Induction Therapy in Childhood Acute Lymphoblastic Leukemia: A Systematic Review and Meta-Analysis. Leukemia (2011) 25(8):1232–8. doi:10.1038/leu.2011.84
33. Kearney, SL, DahlbergLevy, SEDE, Voss, SD, Sallan, SE, and Silverman, LB. Clinical Course and Outcome in Children with Acute Lymphoblastic Leukemia and Asparaginase-Associated Pancreatitis. Pediatr Blood Cancer (2009) 53:162–7. doi:10.1002/pbc.22076
34. Junquera Alonso, E, Seoane Blanco, L, and Cano Calderero, FX. Bortezomib-induced Acute Pancreatitis, an Uncommon Adverse Event. Rev Esp Enferm Dig (2021) 113(1):77. doi:10.17235/reed.2020.7120/2020
35. Brulc, E, Seehaus, C, Schutz, N, and Fantl, D. Management of Pancreatitis Related to Bortezomib Treatment: Report of Two Cases. Hematol Transfus Cel Ther (2018) 40(4):382–4. doi:10.1016/j.htct.2018.03.003
36. Halalsheh, H, Bazzeh, F, Alkayed, K, Salami, K, and Madanat, F. 6-Mercaptopurine-induced Recurrent Acute Pancreatitis in Children with Acute Lymphoblastic Leukemia/Lymphoma. J Pediatr Hematol Oncol (2013) 35(6):470–2. doi:10.1097/mph.0b013e318271c92f
37. Zerra, P, Bergsagel, J, Keller, FG, Glen, L, and Pauly, M. Maintenance Treatment with Low-Dose Mercaptopurine in Combination with Allopurinol in Children with Acute Lymphoblastic Leukemia and Mercaptopurine-Induced Pancreatitis. Pediatr Blood Cancer (2016) 63(4):712–5. doi:10.1002/pbc.25841
38. McGrail, LH, Sehn, LH, Weiss, RB, Robson, MR, Antin, JH, Byrd, JC, et al. Pancreatitis during Therapy of Acute Myeloid Leukemia: Cytarabine Related? Ann Oncol (1999) 10(11):1373–6. doi:10.1023/a:1008342320532
39. Sastry, J, Young, S, and Shaw, P. Acute Pancreatitis Due to Tacrolimus in a Case of Allogeneic Bone Marrow Transplantation. Bone Marrow Transpl (2004) 33:867–8. doi:10.1038/sj.bmt.1704429
40. Xu, J, Xu, L, Wei, X, Li, X, and Cai, M. A Case Report: Acute Pancreatitis Associated with Tacrolimus in Kidney Transplantation. BMC Nephrol (2019) 20:209. doi:10.1186/s12882-019-1395-x
41. Stock, W, Douer, D, DeAngelo, DJ, Arellano, M, Advani, A, Damon, L, et al. Prevention and Management of Asparaginase/pegasparaginase-Associated Toxicities in Adults and Older Adolescents: Recommendations of an Expert Panel. Leuk Lymphoma (2011) 52(12):2237–53. doi:10.3109/10428194.2011.596963
42. Abu-El-Haija, M, Kumar, S, Quiros, JA, Balakrishnan, K, Barth, B, Bitton, S, et al. Management of Acute Pancreatitis in the Pediatric Population: A Clinical Report From the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Pancreas Committee. J Pediatr Gastroenterol Nutr (2018) 66(1):159–76. doi:10.1097/MPG.0000000000001715
43. Isenmann, R, Rünzi, M, Kron, M, Kahl, S, Kraus, D, Jung, N, et al. Prophylactic Antibiotic Treatment in Patients with Predicted Severe Acute Pancreatitis: A Placebo-Controlled, Double-Blind Trial. Gastroenterology (2004) 126(4):997–1004. doi:10.1053/j.gastro.2003.12.050
44. García-Barrasa, A, Borobia, FG, Pallares, R, Jorba, R, Poves, I, Busquets, J, et al. A Double-Blind, Placebo-Controlled Trial of Ciprofloxacin Prophylaxis in Patients with Acute Necrotizing Pancreatitis. J Gastrointest Surg (2009) 13(4):768–74. doi:10.1007/s11605-008-0773-7
45. Dambrauskas, Z, Gulbinas, A, Pundzius, J, and Barauskas, G. Meta-analysis of Prophylactic Parenteral Antibiotic Use in Acute Necrotizing Pancreatitis. Medicina (Kaunas) (2007) 43(4):291. doi:10.3390/medicina43040036
46. Thu Huynh, V, and Bergeron, S. Asparaginase Toxicities: Identification and Management in Patients with Acute Lymphoblastic Leukemia. Clin J Oncol Nurs (2017) 21(5):E248–E259. doi:10.1188/17.CJON.E248-E259
47. Burke, PW, Hoelzer, D, Park, JH, Schmiegelow, K, and Douer, D. Managing Toxicities with Asparaginase Based Therapies in Adult ALL: Summary of an ESMO Open– Cancer Horizons Roundtable Discussion. ESMO Open (2020) 5:e000858. doi:10.1136/esmoopen-2020-000858
48. Oak, E, and Bartlett, NL. A Safety Evaluation of Brentuximab Vedotin for the Treatment of Hodgkin Lymphoma. Expert Opin Drug Saf (2016) 15:875–82. doi:10.1080/14740338.2016.1179277
49. Carson, KR, Newsome, SD, Kim, EJ, Wagner-Johnston, ND, von Geldern, G, Moskowitz, CH, et al. Progressive Multifocal Leukoencephalopathy Associated with Brentuximab Vedotin Therapy: A Report of 5 Cases from the Southern Network on Adverse Reactions (SONAR) Project. Cancer (2014) 120:2464–71. doi:10.1002/cncr.28712
50. Berger, JR, Aksamit, AJ, Clifford, DB, Davis, L, Koralnik, IJ, Sejvar, JJ, et al. PML Diagnostic Criteria: Consensus Statement from the AAN Neuroinfectious Disease Section. Neurology (2013) 80(15):1430–8. doi:10.1212/WNL.0b013e31828c2fa1
51.EMA Europa. Adcetris. [Internet] (2022). Available from: https://www.ema.europa.eu/en/documents/product-information/adcetris-epar-product-information_en.pdf (Accessed February 23, 2022).
Keywords: Hodgkin lymphoma, pediatric oncology, brentuximab vedotin, acute pancreatitis, side effects
Citation: Truszkowska E, Andrzejewska M, Szymańska C, Wziątek A and Derwich K (2022) Case Report: Brentuximab Vedotin Associated Acute Pancreatitis in a Pediatric Hodgkin Lymphoma Patient: Case Report and Literature Review. Pathol. Oncol. Res. 28:1610445. doi: 10.3389/pore.2022.1610445
Received: 16 March 2022; Accepted: 15 July 2022;
Published: 12 August 2022.
Edited by:
Edit Bardi, St. Anna Kinderspital, AustriaCopyright © 2022 Truszkowska, Andrzejewska, Szymańska, Wziątek and Derwich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katarzyna Derwich, a2RlcndpY2hAdW1wLmVkdS5wbA==
 Ewelina Truszkowska
Ewelina Truszkowska Marta Andrzejewska
Marta Andrzejewska Cyntia Szymańska
Cyntia Szymańska Agnieszka Wziątek2
Agnieszka Wziątek2