- 1Department of Neuro-Oncology, Guangdong Sanjiu Brain Hospital, Guangzhou, China
- 2State Key Laboratory of Translational Medicine and Innovative Drug Development, Jiangsu Simcere Diagnostics Co., Ltd., Nanjing, China
Adult brainstem gliomas are rare central nervous system tumors that represent a heterogeneous group of tumors. Somatic IDH mutations are uncommon in adult brainstem gliomas and there are few relevant clinical studies. Here, we reported five patients with IDH1 mutations associated with brainstem gliomas, including four cases of IDH1 R132H mutations and one case of R132G mutation. All patients were treated with focal intensity-modulated radiation therapy (IMRT) with concurrent temozolomide (TMZ). One patient died, one relapsed, and three survived to date. All these cases carried a pathogenic variant of TP53, among whom 1 harbored ATRX mutation and 1 had H3K27M mutation. Moreover, we also found some genes related to a worse prognosis, such as CDK4/6 amplification. These findings demonstrate that the specific characteristics of IDH-mutant brainstem gliomas should be considered in diagnostic workflows to make therapeutic regimens and improve the prognosis.
Introduction
Brainstem gliomas are relatively rare in the central nervous system, only representing 1–2% of all adult brain tumors [1]. Adult brainstem gliomas represent a heterogeneous group of tumors arising from the midbrain (12–15%), pons (60–63%), or medulla oblongata (25%) [2]. Based on clinicopathologic and magnetic resonance imaging (MRI) characteristics, they are classified into diffuse, intrinsic focal, extrinsic focal, and cervicomedullary types [3]. In contrast to pediatric gliomas, isocitrate dehydrogenase (IDH) mutations are more frequent in adult low-grade glioma [4]. However, IDH-mutant gliomas arising from the brainstem are exceedingly rare in adults and children. In this study, we described the clinicopathological and molecular characteristics of the five adult patients with IDH-mutant brainstem gliomas (Table 1 and Supplementary Table S1) in detail.
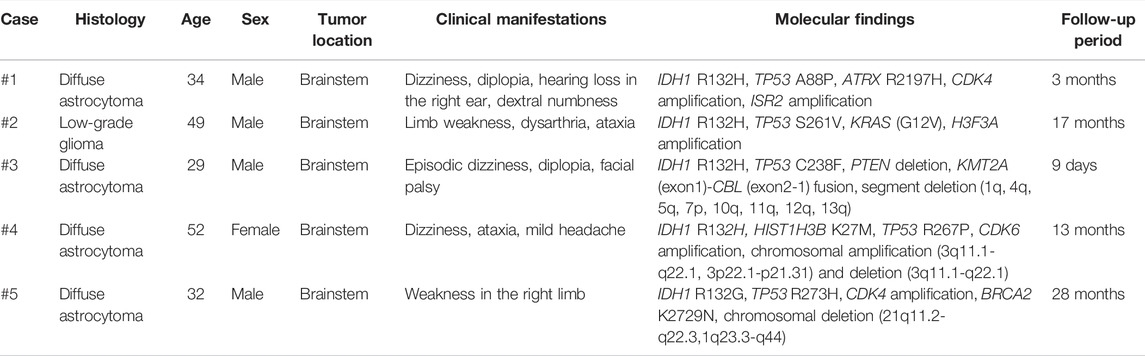
TABLE 1. Clinocopathological and molecular characteristics of the five adults with brainstem gliomas.
Case Description
Case #1 was a 34-year-old man who developed dizziness, diplopia, hearing loss in the right ear, and dextral numbness in December 2018. The brain MRI indicated obvious swelling and abnormal signals in the cervical medullary segment, brainstem, and bilateral bridge arms in May 2019, considering the possibility of high-grade glioma (Figures 1A–C), thus the brainstem space-occupying excision was performed. Histology revealed a diffuse growth in the tumor with moderately increased cell density (Figure 1D). By immunohistochemistry (IHC), IDH1-positive tumor cells (Figure 1E) and Ki-67 of 5% were observed (Figure 1F). Fluorescence in situ hybridization (FISH) analysis showed that chromosomes 1p and 19q were not deleted, but IDH1 R132H mutation was confirmed by Sanger sequencing. The targeted next-generation sequencing (NGS) using a panel of 131 genes and 4 chromosomes [5] (131 + 4 panel, Supplementary Table S2) further identified mutations in IDH1, TP53, and ATRX, CDK4 amplification, and IRS2 amplification. In combination with histopathology and molecular analysis, the patient was diagnosed with astrocytoma, IDH-mutant WHO grade II. He was given anti-epileptic therapies due to pre- and post-operative epileptic seizures and got better after focal intensity-modulated radiation therapy (IMRT, DT 54Gy/27f) with concurrent temozolomide (TMZ, 120 mg). In March 2021, the tumor relapsed due to the presence of enlarged lesions in the callosum-septum pellucidum area and multiple lesions as before, and then concurrent radiochemotherapy and symptomatic treatment were applied. Approximately 20 days after treatment, the patient was discharged from the hospital and continued to receive radiochemotherapy as an outpatient.
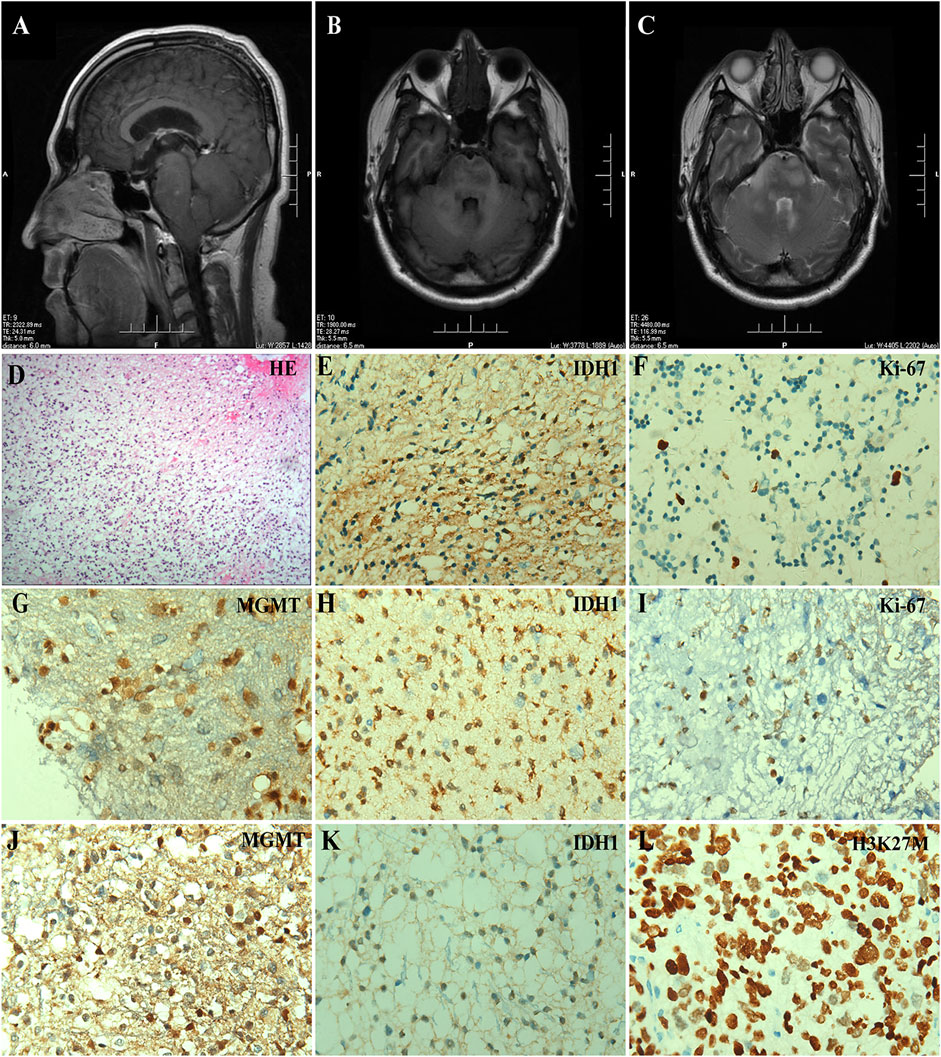
FIGURE 1. IDH-mutant brainstem gliomas in adult patients. (A–C) Preoperative MRI scanning of Case #1, including a plain scan of T1-weighted (A) and T2-weighted (B) images in axial views and a contrast-enhanced scan of T1-weighted images in sagittal view (C). The arrowhead indicated the lesion in the brainstem. (D–F) Histologic and immunohistochemical features of the tumor from Case #1. (D) HE staining, (E) IDH1 R132H, and (F) Ki-67 immunostaining (×400). (G–I) Immunohistochemical staining of the tumor from Case #2, including (G) MGMT, (H) IDH1 R132H, and (I) Ki-67 (×400). (J–L) Immunohistochemical staining of the tumor from Case #4, including (J) MGMT, (K) IDH1 R132H, and (L) H3K27M (×400).
Case #2 was a 49-year-old man with limb weakness, dysarthria, ataxia, and sometimes dizziness. Brain MRI and magnetic resonance spectrum (MRS) showed a brainstem lesion, thus the brainstem biopsy was conducted. Low-grade glioma was considered. Tumor cells were positive for O-6-methylguanine-DNA methyltransferase (MGMT) and IDH1, with a proliferation activity of 2% for Ki-67 (Figures 1G–I). IDH1, TP53, and KRAS mutations and H3F3A amplification were detected using the targeted 131 + 4 panel. The patient was treated with focal IMRT (DT 54Gy/27f) with concurrent TMZ (75 mg/m2) and TMZ maintenance therapy after hospital discharge. The patient survived, with weakness in the lower limbs.
Case #3 was a 29-year-old man with episodic dizziness, diplopia, and facial palsy for 1 month. He received a robot-guided biopsy due to brain MRI findings of a space-occupying lesion between the brainstem and the right cerebellar hemisphere. Microscopically, the tumor showed diffuse invasive growth with moderately increased cell density. Tumor cells were positive for MGMT and IDH1, with a proliferation activity of 4% for Ki-67. Through the targeted 131 + 4 panel, it was found that IDH1 and TP53 mutations, PTEN deletion, KMT2A-CBL fusion, and segment deletion of the chromosome. Based on these findings, the patient was diagnosed with astrocytoma, IDH-mutant WHO grade II, and was treated with focal IMRT (DT 54Gy/30f) with concurrent TMZ (75 mg/m2). Three weeks later, the peritumoral edema improved. However, 2 months after treatment, the computed tomography (CT) indicated a slowly increasing brainstem hemorrhage. Family members refused for the patient to be transferred to the intensive care unit, and he was dead 9 days later from brainstem hemorrhage.
Case #4 was a 52-year-old woman experiencing dizziness, ataxia, and mild headache in 2019. Brain CT, MRI, and MRS all suggested space-occupying neoplasms in the brainstem in November 2020. Under general anesthesia, brainstem glioma excision, decompressive craniectomy, and extra ventricular drainage were performed. Tumor cells were positive for MGMT, IDH1, and H3K27M (Figures 1J–L), with Ki-67 of 7%. Interestingly, the targeted 131 + 4 panel revealed mutations in IDH1, H3K27M, and TP53, CDK6 amplification, as well as chromosomal amplification (3q11.1-q22.1, 3p22.1-p21.31) and deletion (3q11.1-q22.1). She was diagnosed with astrocytoma, IDH1-mutant, H3K27M-mutant WHO grade II. Due to economic factors, the patient refused chemotherapy and received conformal radiotherapy. She was discharged from the hospital for personal reasons and has survived to date.
Case #5 was a 32-year-old man with weakness in the right limb in December 2018. Brain MRI in May 2019 indicated a space-occupying lesion in the medulla oblongata, considered a low-grade glioma. After the brainstem glioma excision, dura mater reparation, and cranioplasty, the patient was diagnosed with astrocytoma, IDH-mutant WHO grade II based on histopathology and molecular analysis which showed mutations in IDH1 and TP53, CDK4 amplification, BRCA2, and chromosomal deletion (21q11.2-q22.3,1q23.3-q44) using the targeted 131 + 4 panel. Focal IMRT (DT 40Gy/20f) with concurrent TMZ (120 mg) was used. About 1 month after treatment, the patient’s symptoms improved, and he left the hospital following the fourth cycle of TMZ chemotherapy (340 mg). According to the doctor’s advice, the subsequent fifth and sixth cycles of TMZ chemotherapy (340 mg) were used. The disease was stable 2 years after treatment.
Discussion
IDH mutations are uncommon in brainstem gliomas. The majority (90%) of astrocytomas and oligodendrogliomas carry a canonical IDH1 R132H mutation [6], which can be detected by IDH1 (R132H), while other mutations should be detected through the sequencing involving IDH1 and IDH2 genes. In our report, the tumor cells from four cases showed positive for IDH1 (R132H), which were confirmed by sequencing techniques, and a noncanonical IDH1 R132G mutation was identified in Case #5 by Sanger sequencing. All these cases carried a pathogenic variant of TP53, further suggesting the importance of TP53 mutations in astrocytoma. Previous studies indicated that the TP53 rs4968187 variant was associated with the risk of developing astrocytoma, and the TP53 rs78378222 variant was significantly connected with a higher risk of glioblastoma [7, 8].
By analyzing the genetic alterations in diffuse low‐grade gliomas, Aoki et al. found that altered RB pathway genes including CDKN2A and CDK4 could be independent predictors of poor survival in IDH-mutant astrocytomas [9]. Among these five patients, however, no CDKN2A/B deletion was identified, but CDK4/6 amplification was found in Cases #1, #4, and #5. Selective inhibition of CDK4/6 was reported to suppress the tumor growth and may enhance the sensitivity of glioma cells to TMZ, suggesting that CDK4/6 inhibition may be a favorable treatment strategy for glioma and overcome TMZ resistance [10]. Currently, abemaciclib and ribociclib, two kinds of CDK4/6 inhibitors, are being explored in phase I trials for pediatric and adult patients with glioblastoma, although palbociclib, a dual CDK4/6 inhibitor, is ineffective in the treatment of recurrent glioblastoma [11–13].
In our report, the tumor cells of three patients were positive for MGMT, whose promoter methylation (Supplementary Figure S1) is considered a strong prognostic biomarker in pediatric and adult glioblastoma patients treated with TMZ [14, 15]. In Case #4, a H3K27M mutation was identified. This mutation could lead to a unique relatively homogenous phenotype with key epigenetic, genetic, and clinical significance. Notably, mutations in H3K27M and IDH1 may be mutually exclusive. Compared with other IDH-mutant infratentorial gliomas, two patients with noncanonical IDH1 R132C and H3K27M mutations showed shorter overall survival [16].
ATRX is rarely mutated in adults with primary high-grade gliomas, but is more frequent in younger adults with lower-grade gliomas [17]. In our report, ATRX mutation was identified in Case #1 who was a young adult with lower-grade gliomas. There are studies suggesting ATRX mutations are associated with better prognosis in IDH-mutant, low-grade glioma patients without 1p/19q co-deletion [18, 19]. Although no chromosome 1p/19q deletion was found in this report, multiple specific chromosomal alterations were identified in three lower-grade gliomas, including on chromosome 1q, 4q, 5q, 7p, 10q, 11q, 12q, 13q deletions, and more. A previous study showed that astrocytomas progressed rapidly to glioblastoma, with short survival intervals and increased frequencies of 9p, 10q, and 13q deletions [20]. Additionally, the KMT2A-CBL fusion previously reported in acute leukemia was first identified in brainstem gliomas. The breakpoints of the KMT2A and CBL genes in Case #3 were different from those in acute leukemia (Figure 2), which should be validated by other methods like RNA-seq.
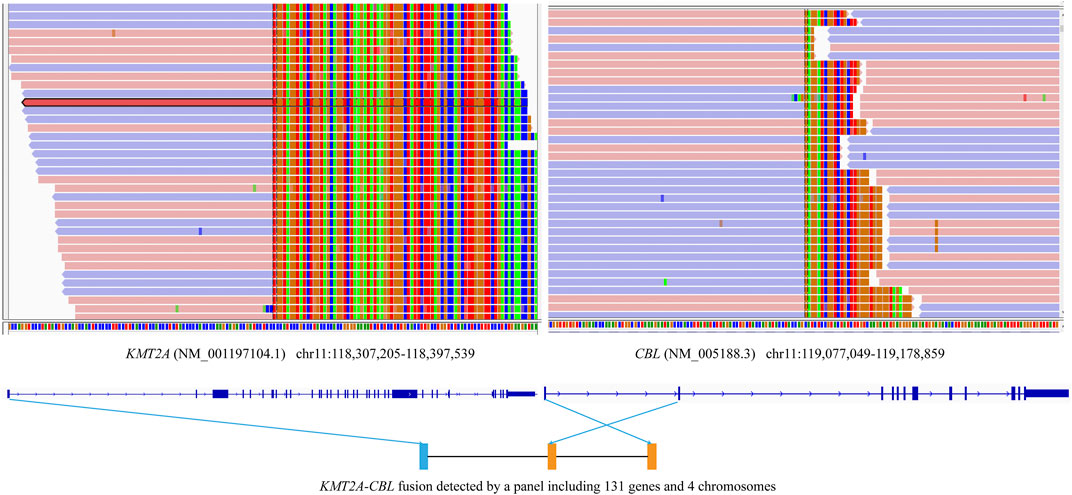
FIGURE 2. Schematic diagrams of the novel KMT2A-CBL fusion. The KMT2A-CBL fusion between KMT2A exon 1 and CBL exon 2 in the tumor of Case #3 using a panel of 131 genes and 4 chromosomes.
Conclusion
These typical cases suggest that adult IDH1-mutant brainstem gliomas have different clinicopathological and genetic characteristics, some of which may be associated with poor prognosis. These findings demonstrate that the specific characteristics of IDH-mutant brainstem gliomas should be considered in diagnostic workflows to make therapeutic regimens and improve the prognosis.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
JFZ: Conception and design of the study, writing-original draft; MYL, YN, SQL, and JJZ: Data acquisition and investigation; FRD and XZ: Data analysis and methodology; CS and LBC: Conception and design of the study, supervision, and writing-editing and reviewing.
Conflict of Interest
Authors YN, FD, XZ, and CS were employed by the Jiangsu Simcere Diagnostics Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the laboratory technicians from Simcere Diagnostics, Co. Ltd. (Nanjing, China), for next-generation sequencing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2022.1610408/full#supplementary-material
References
1. Ostrom, QT, Gittleman, H, Fulop, J, Liu, M, Blanda, R, Kromer, C, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol (2015) 17(Suppl. 4):iv1–iv62. doi:10.1093/neuonc/nov189
2. Eisele, SC, and Reardon, DA. Adult Brainstem Gliomas. Cancer (2016) 122:2799–809. doi:10.1002/cncr.29920
3. Salmaggi, A, Fariselli, L, Milanesi, I, Lamperti, E, SilvAni, A, Bizzi, A, et al. Natural History and Management of Brainstem Gliomas in Adults. A Retrospective Italian Study. J Neurol (2008) 255:171–7. doi:10.1007/s00415-008-0589-0
4. Johnson, DR, Brown, PD, Galanis, E, and Hammack, JE. Pilocytic Astrocytoma Survival in Adults: Analysis of the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. J Neurooncol (2012) 108:187–93. doi:10.1007/s11060-012-0829-0
5. Wang, Y, Wang, L, Blümcke, I, Zhang, W, Fu, Y, Shan, Y, et al. Integrated Genotype-Phenotype Analysis of Long-Term Epilepsy-Associated Ganglioglioma. Brain Pathol (2022) 32:e13011. doi:10.1111/bpa.13011
6. Hartmann, C, Meyer, J, Balss, J, Capper, D, Mueller, W, Christians, A, et al. Type and Frequency of IDH1 and IDH2 Mutations Are Related to Astrocytic and Oligodendroglial Differentiation and Age: a Study of 1, 010 Diffuse Gliomas. Acta Neuropathol (2009) 118:469–74. doi:10.1007/s00401-009-0561-9
7. Carlos-Escalante, JA, Gómez-Flores-Ramos, L, Bian, X, Perdomo-Pantoja, A, de Andrade, KC, Mejia-Perez, SI, et al. Landscape of Germline Genetic Variants in AGT, MGMT, and TP53 in Mexican Adult Patients with Astrocytoma. Cell Mol Neurobiol (2021) 41:1285–97. doi:10.1007/s10571-020-00901-7
8. Wang, Z, Rajaraman, P, Melin, BS, Chung, CC, Zhang, W, McKean-Cowdin, R, et al. Further Confirmation of Germline Glioma Risk Variant Rs78378222 in TP53 and its Implication in Tumor Tissues via Integrative Analysis of TCGA Data. Hum Mutat (2015) 36:684–8. doi:10.1002/humu.22799
9. Aoki, K, Nakamura, H, Suzuki, H, Matsuo, K, Kataoka, K, Shimamura, T, et al. Prognostic Relevance of Genetic Alterations in Diffuse Lower-Grade Gliomas. Neuro Oncol (2018) 20:66–77. doi:10.1093/neuonc/nox132
10. Cao, Y, Li, X, Kong, S, Shang, S, and Qi, Y. CDK4/6 Inhibition Suppresses Tumour Growth and Enhances the Effect of Temozolomide in Glioma Cells. J Cel Mol Med (2020) 24:5135–45. doi:10.1111/jcmm.15156
11.University of Texas Southwestern Medical Center. Pilot Study of Abemaciclib with Bevacizumab in Recurrent Glioblastoma Patients with Loss of CDKN2A/B or Gain or Amplification of CDK4/6. Available online: https://ClinicalTrials.gov/show/NCT04074785 (Accessed May 16, 2020).
12.Barrow Neurological Institute. A Phase 0/II Study of Ribociclib (LEE011) in Combination with Everolimus in Preoperative Recurrent High-Grade Glioma Patients Scheduled for Resection. Available online: https://ClinicalTrials.gov/show/NCT03834740 (Accessed May 16, 2020).
13. Taylor, JW, Parikh, M, Phillips, JJ, James, CD, Molinaro, AM, Butowski, NA, et al. Phase-2 Trial of Palbociclib in Adult Patients with Recurrent RB1-Positive Glioblastoma. J Neurooncol (2018) 140:477–83. doi:10.1007/s11060-018-2977-3
14. Wick, W, Weller, M, van den Bent, M, Sanson, M, Weiler, M, von Deimling, A, et al. MGMT Testing-Tthe Challenges for Biomarker-Based Glioma Treatment. Nat Rev Neurol (2014) 10:372–85. doi:10.1038/nrneurol.2014.100
15. Hegi, ME, Diserens, AC, Gorlia, T, Hamou, MF, de Tribolet, N, Weller, M, et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N Engl J Med (2005) 352:997–1003. doi:10.1056/NEJMoa043331
16. Banan, R, Stichel, D, Bleck, A, Hong, B, Lehmann, U, Suwala, A, et al. Infratentorial IDH-Mutant Astrocytoma Is a Distinct Subtype. Acta Neuropathol (2020) 140:569–81. doi:10.1007/s00401-020-02194-y
17. Suzuki, H, Aoki, K, Chiba, K, Sato, Y, Shiozawa, Y, Shiraishi, Y, et al. Mutational Landscape and Clonal Architecture in Grade II and III Gliomas. Nat Genet (2015) 47:458–68. doi:10.1038/ng.3273
18. Leeper, HE, Caron, AA, Decker, PA, Jenkins, RB, Lachance, DH, Giannini, C, et al. IDH Mutation, 1p19q Codeletion and ATRX Loss in WHO Grade II Gliomas. Oncotarget (2015) 6:30295–305. doi:10.18632/oncotarget.4497
19. Wiestler, B, Capper, D, Holland-Letz, T, Korshunov, A, von Deimling, A, Pfister, SM, et al. ATRX Loss Refines the Classification of Anaplastic Gliomas and Identifies a Subgroup of IDH Mutant Astrocytic Tumors with Better Prognosis. Acta Neuropathol (2013) 126:443–51. doi:10.1007/s00401-013-1156-z
Keywords: prognosis, IDH1 mutation, molecular features, temozolomide, brainstem glioma
Citation: Zhou J, Lai M, Ni Y, Li S, Zhen J, Du F, Zhang X, Song C and Cai L (2022) Case Report: Clinicopathological and Genetic Features of IDH-Mutant Brainstem Glioma in Adults: Report of Five Cases. Pathol. Oncol. Res. 28:1610408. doi: 10.3389/pore.2022.1610408
Received: 01 March 2022; Accepted: 30 June 2022;
Published: 04 August 2022.
Edited by:
Anna Sebestyén, Semmelweis University, HungaryCopyright © 2022 Zhou, Lai, Ni, Li, Zhen, Du, Zhang, Song and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Song, Y2hhby5zb25nQHNpbWNlcmVkeC5jb20=; Linbo Cai, Y2FpbGluYm9nekAxMjYuY29t
 Jiangfen Zhou1
Jiangfen Zhou1 Chao Song
Chao Song Linbo Cai
Linbo Cai