Abstract
Non-Hodgkin lymphomas comprise a heterogeneous group of malignancies, with a wide scope of clinical, radiological and histological presentations. In this paper, a case is presented of a 59-year-old white male with an infraorbital follicular B-cell lymphoma, which appeared as a painless mass in the left cheek. The lymphoma achieved spontaneous remission five and a half months after his diagnostic incision biopsy. The literature is reviewed, focusing on this rare site of presentation and spontaneous remission. In literature, only four cases have been reported with a follicular B-cell lymphoma of the cheek or infraorbital region, and only 26 cases of spontaneous remission of an extracranial non-Hodgkin lymphoma in the head and neck region have been described. To the authors’ best knowledge, this is the first time spontaneous remission of an infraorbital follicular lymphoma could be observed. The nature of the processes inducing spontaneous remission remains obscure. It is important to recognize this phenomenon as this might prevent unnecessary treatment.
Introduction
Non-Hodgkin lymphomas (NHL) comprise a heterogeneous group of malignancies, generally consisting of T-cell and B-cell neoplasms. Follicular lymphoma, a NHL, is the most common indolent lymphoma and accounts for around 10–20% of all lymphomas in western countries [1].
Spontaneous remission of cancer is a rare and intriguing phenomenon, with an unknown underlying mechanism, still being a subject of debate for clinicians, radiologists, pathologists, hematologists and researchers. Spontaneous remission appears in one case per 80,000–100,000 and occurs sporadically in patients with NHL [2].
The cheek and infraorbital area are uncommon sites for lymphomas. Spontaneous remission of follicular lymphoma located in this area is even more exceptional and has never been reported to our knowledge. This paper describes a well-documented case of a patient diagnosed with an infraorbital localized follicular lymphoma, following spontaneous remission within months.
Case Presentation
A 59-year-old white male was referred to the University Hospitals Leuven, Department of Oral and Maxillofacial Surgery for evaluation of a left infraorbital mass. The mass presented two months earlier as a small nodule, rapidly increasing in size over a period of two days. Because the swelling persisted and even increased in size, the patient consulted his general practitioner. Consecutively, an ultrasound and a Magnetic Resonance Imaging (MRI) scan (Figure 1A) of the head and neck were performed. The patient was referred to the Oral and Maxillofacial Surgery Department, as these investigations suggested a schwannoma of the left infraorbital nerve.
FIGURE 1
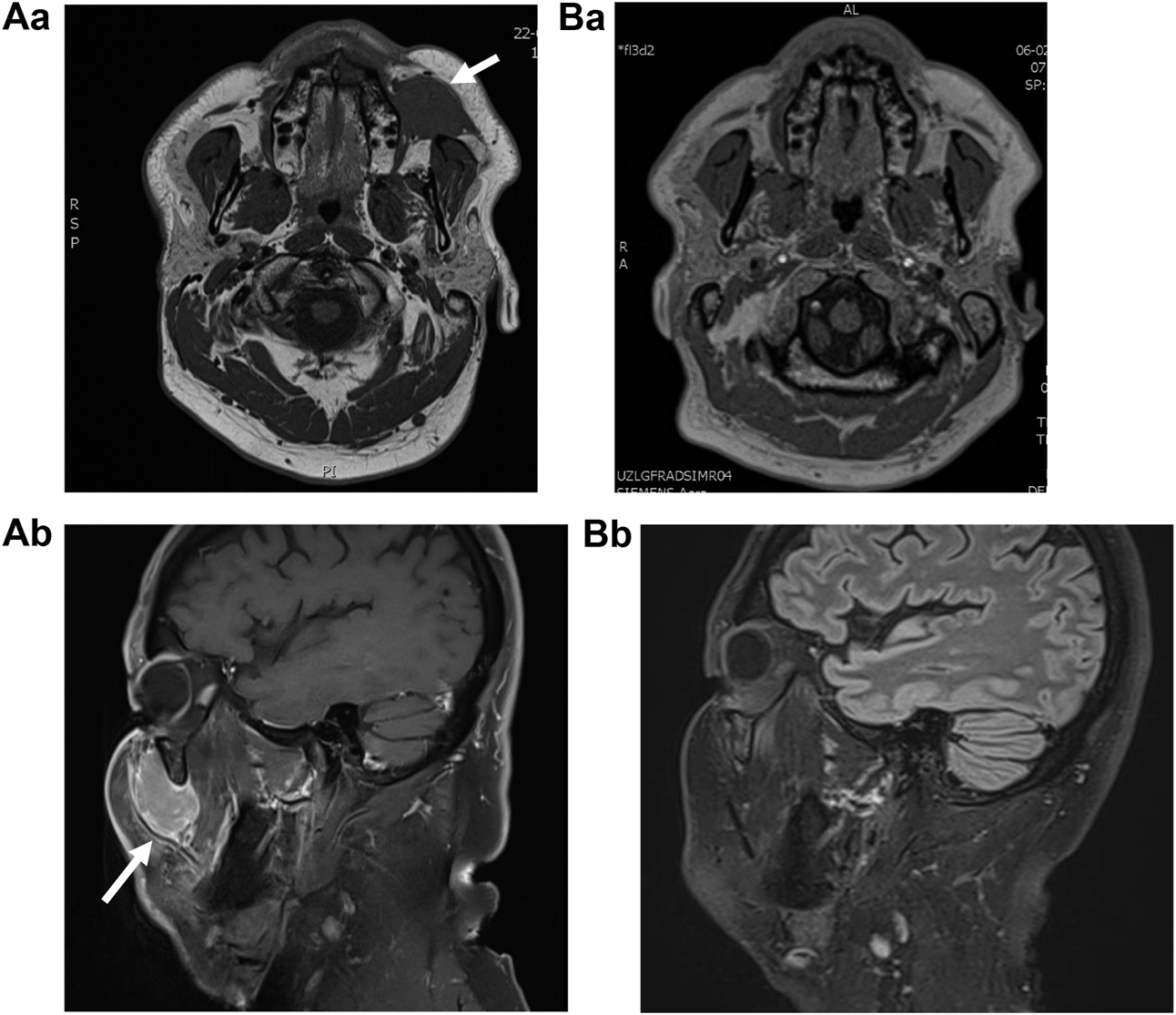
MRI (Magnetic Resonance Imaging) (A) at the moment of first presentation, depicting a volume of 28 mm × 29 mm × 42 mm (AP × ML × CC) and (B) 5.5 months later, after an incision biopsy was performed. Aa, This T2 weighted image depicts a well-described, mass of intermediate intensity at the buccinator space. Ba, T2 weighted image depicting the absence of the lesion, indicative for tumor remission. Ab, T1 weighted image with Gadolinium contrast, showing expansion to the infraorbital region. Contrastcaptation is moderate. Bb, T1 weighted image depicting the absence of the lesion.
The patient’s medical history consisted of recurrent vestibulopathy, vitreous floaters, protein S deficiency, and two episodes of popliteal vein thrombosis, in 1999 and 2006. He was taking Atorvastatin and Rivaroxaban daily. B symptoms were absent. Clinical examination revealed an infraorbital, mobile mass measuring 4 × 2 cm (Appendix 1: PDF VECTRA). Quantitative Sensory Testing indicated a very subtle hypo-aesthesia of the left infraorbital nerve. Laboratory testing showed no abnormalities. An incision biopsy (0.8 × 1 cm) was performed under local anesthesia. During this procedure, a well-described, encapsulated mass was encountered (Figure 2).
FIGURE 2
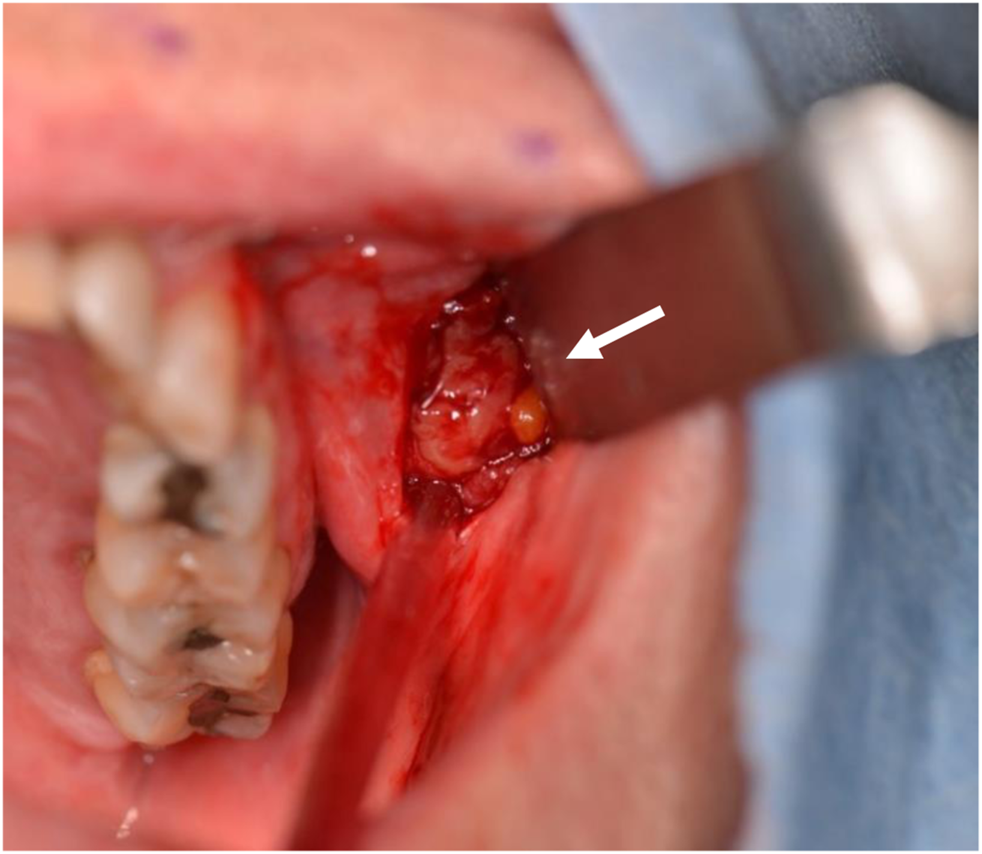
Biopsy. The mass presented as a well-described, encapsulated lesion.
Histological assessment was performed by the Pathology Department of University Hospitals Leuven, and reviewed by the Pathology Department of Lausanne University Hospital (CHUV). It showed a disturbed lymph node architecture, with vague nodules organized in a back-to-back manner (Figure 3). The follicles were composed of a mixture of centrocytes and centroblasts (<15/HPF), and numerous small T-lymphocytes, but no tingible body macrophages. The B-cells in the nodular infiltrates expressed CD20+, PAX5+, CD10+, Bcl6+, Ki 67+ (<20%) and overexpressed BCL2. The nodules were supported by CD21+ follicular dendritic networks. In between the B-cells in the follicle centers, there were increased numbers of CD3+, PD1+, CXCL13+, weak ICOS+ follicular helper T-cells. Both heavy and light chain gene rearrangements, as well as T-cell receptor beta and gamma gene rearrangements were found. FISH, using probe BCL2 (DC BA)[18q21, Vysis], uncovered a rearrangement of locus BCL2/18q21 in the B-cells. These findings yielded the diagnosis of a B-cell follicular lymphoma, grade 1-2, BCL-2 positive, with BCL2-gene rearrangement, with numerous intrafollicular follicular T-helper cells. The florid TFH cell (T-follicular helper cell) infiltrate is unusual but we would favor that it is reactive in nature. The presence of small T-cell clones in the presence of B-cell lymphomas are likely indicative of a prominent clonal component in the tumor, and do not warrant a diagnosis of composite follicular T-cell lymphoma.
FIGURE 3
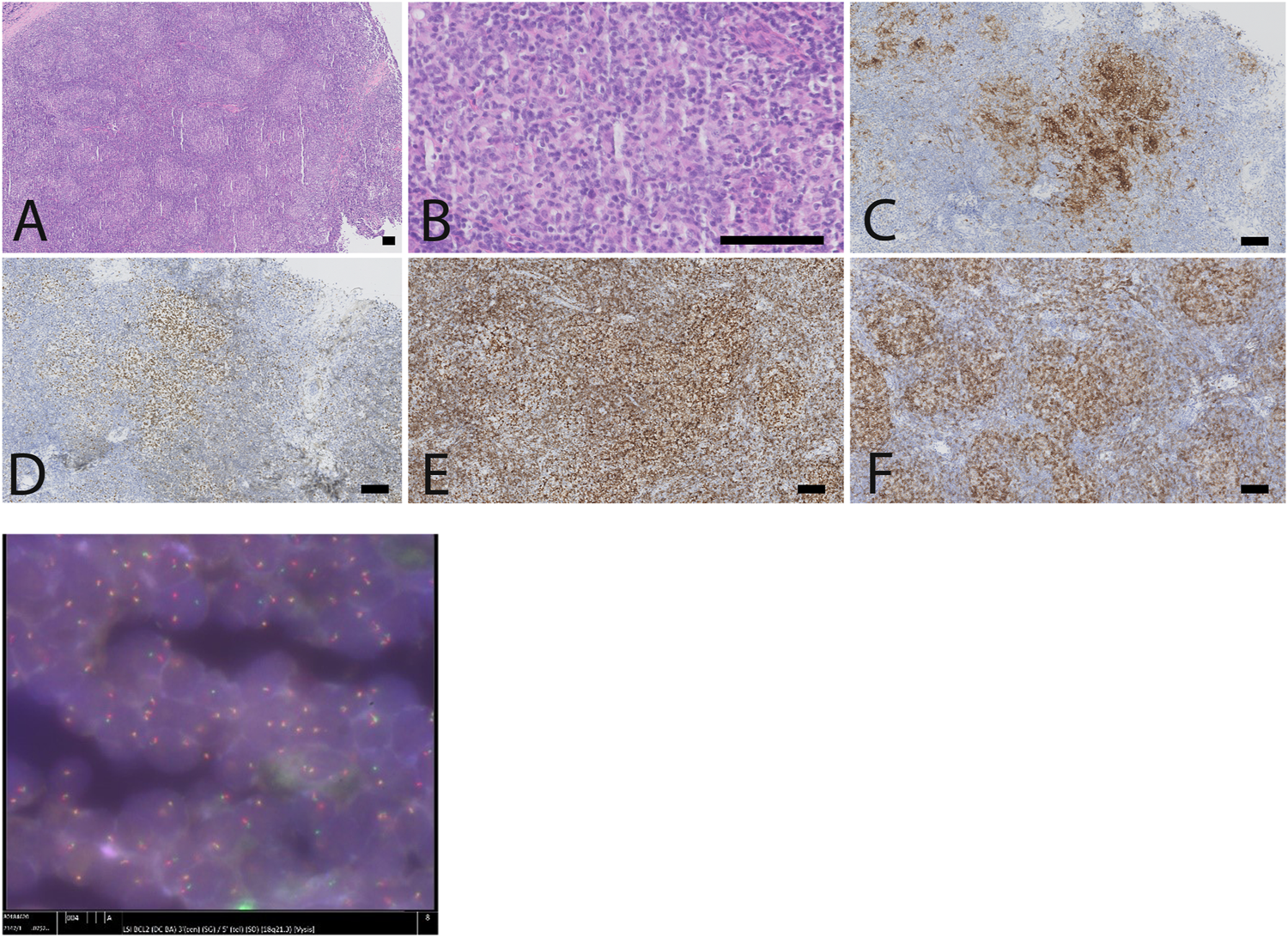
Lymph node biopsy, showing a follicular lymphoma with brisk TFH response and BCL2-rearrangement. (A) low power view showing a disturbed lymph node architecture, with numerous small follicles arranged in a back-to-back fashion. (B). The follicle centers are composed predominantly of small centrocytes and scattered centroblasts (less than 15/high power field), in the absence of tangible body macrophages. The B-cells in de follicles express CD10 (C), BCL6 (D), overexpress BCL2 (E) and are intermingled with numerous follicular T-helper cells, as illustrated by a immunostaining against PD1 (F). (G) FISH highlights the presence of a BCL2-rearrangement, corresponding to the BCL2 overexpression in the follicles, as illustrated in the anti-BCL2 immunostain (E) (scale bar: 100 µm).
Staging included clinical examination, and an 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) computed tomography (CT) scan, and revealed no other lymphadenopathies, nor FDG-avid lesions. Because of technical problems, the CT scan generated unusable images. As the infraorbital hypo-aesthesia persisted, further imaging was ordered which revealed a complete and spontaneous remission of the tumor mass (5.5 months after the initial MRI) (Figure 1B). Clinical examination was consistent with these findings. Bone marrow trephine and aspirate were negative. The tumor showed no recurrence during clinical and biochemical follow-up, six months after its spontaneous remission. The patient will receive a clinical and biochemical follow-up after one year.
Discussion
Malignant lymphoma accounts for 5% of all head and neck cancers and an estimated 2.5% of all malignant lymphomas arise at the level of the cheek [3, 4]. Follicular lymphoma of the cheek mostly occurs as a parotid gland tumor [3, 5]. Apart from this parotid localization, follicular lymphomas are very rare at the level of the cheek. To the authors’ best knowledge, only four cases of a non-salivary gland, infraorbital or buccal localization of follicular lymphoma are reported in English literature (Table 1).
TABLE 1
| Case no | Author | Year | Sex/age | Tumor | Localization | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|
| 1 | Farquhar et al. [25] | 2014 | F/66 Y | Follicular lymphoma | Cheek | Steroids for unrelated asthma, with spontaneous remission of the tumor | 2 W |
| 2 | Hardin et al. [26] | 2018 | M/71 Y | Follicular lymphoma | Cheek, skin | — | — |
| 3 | Dhadlie et al. [27] | 2018 | F/70 Y | Follicular lymphoma, low-grade | Cheek, subcutaneous | — | — |
| 4 | Thaker et al. [28] | 2019 | F/65Y | Follicular lymphoma, low-grade | Cheek, superficial to the masseter muscle and parotid gland | Surgical excision | — |
| Present case | Peeters et al. | 2020 | M/59 Y | Follicular lymphoma | Buccal space to canine fossa | — | 5.5 M |
Summary of reported cases of follicular lymphoma of the cheek or infraorbital region.
F, female; M, male; W, week; M, month
Lymphomas can originate nodal as well as extranodal. In this case, the presentation and histological findings indicate a nodal localization as 1) lymph nodes are situated in the canine fossa and buccinator space, referred to as ‘forgotten lymph nodes’; 2) an isolated infraorbital lymphoma most frequently arises in the infraorbital lymph node; 3) follicular lymphomas occur more often nodally (76.6%); and 4) BCL-2 expression and rearrangement of locus BCL-2/18q21 indicate a nodal origin rather than an extranodal origin in follicular lymphomas [6–11].
Lymphomas are a heterogeneous group of malignancies with a heterogeneous clinical presentation, often mimicking other malignant and benign lesions. Lymphoma is therefore considered as a ‘great imitator’ [12]. Nodal lymphoma has heterogeneous radiologic features as well [13]. In this case report, the patient presented with a painless mass in the cheek with a very subtle hypo-aesthesia, highlighting the indolent nature of follicular lymphomas [14]. Infraorbital and buccal masses have a variety of etiologies, e.g. odontogenic infection, salivary gland tumor, inflammatory lesion, lipoma, or schwannoma [15, 16]. Due to its acute development, an infectious process was suspected.
Follicular lymphoma is indolent and has a good prognosis, albeit it mostly remains an incurable disease [14]. In this case, it was diagnosed at Ann Arbor stage IA which is rather unusual. Prognosis depends on staging and risk stratification. The Follicular Lymphoma International Prognostic Index score (FLIPI) is widely used to assess the outcome of follicular lymphoma and includes five prognostic factors: age, stage, number of involved nodal areas, serum lactate dehydrogenase and hemoglobin. PET-CT is the standard of care for initial evaluation and staging of lymphoma. PET-CT identified no FDG-avid lesions in this case report. Non-FDG-avidity is associated with indolent lymphomas, and occurs in around 5% of all follicular lymphomas [17]. As the diagnostic lesion did not display FDG-avidity, other associated lesions might have been non-FDG-avid as well. PET detects more nodal sites than CT does, yielding a moderate up-staging in 11% of the nodal follicular lymphomas only assessed by CT; non-FDG-avidity might, therefore, lead to a down-staging of the FLIPI score [18]. In these cases, FLIPI-2 (age, β2‐microglobulin, diameter lymph node, bone marrow involvement and hemoglobin) or PRIMA-PI (β2‐microglobulin, and bone marrow involvement; for de novo follicular lymphoma treated initially with immunochemotherapy) are useful prognostic measures, as they do not include PET results [19, 20].
Spontaneous Remission
Spontaneous remission of cancer is a well-documented, rare phenomenon. The underlying mechanism is still poorly understood and appears to be multifactorial [2]. Several hypotheses might explain the spontaneous remission of cancer, unified by the idea that the innate immune system is triggered and increases its ability to recognize and react on malignant cells [21]. Spontaneous remission occurs mostly in melanomas, hypernephromas and neuroblastomas, and might occasionally take place in lymphomas [22, 23]. To the author’s best knowledge, 26 cases of extracranial, head and neck NHL with spontaneous remission have been reported in English literature (Table 2; excluding relapsing lymphomas and cases that arise in a context of post-transplantation or immunomodulatory-related context, as those latter proliferations are well-known to be likely to resolve or reduce after reduction of the drugs).
TABLE 2
| Case no | Author | Year | Sex/age | Tumour | Localization | Treatment | Other findings | Antecedent prior to remission | Time after antecedent | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Burkitt et al. [29] | 1966 | F/4 Y | ‘African lymphoma' | Maxilla | None | — | Biopsy | 1 Y | 2 Y |
| 2 | Grem et al. [30] | 1986 | F/54 Y | DLBCL | Left vallecula | None | — | Biopsy | — | 4 Y |
| 3 | Poppema et al. [31] | 1988 | M/12 Y | Non-Burkitt's lymphoblastic lymphoma | Oropharynx | None | — | Biopsy | — | 3 Y |
| 4 | Kumamoto et al. [23] | 1994 | F/58 Y | High grade B-cell non-Hodgkin lymphoma | Neck, axilla, inguinal | None | — | Biopsy | 3 W | 2 Y |
| 5 | Savarrio et al. [32] | 1999 | M/77 Y | CD30 + anaplastic large cell lymphoma | Soft palate | None | — | Biopsy | 4 W | Relapse 12 M relapse with DLCBL, cervical region |
| 6 | Koga et al. [33] | 2003 | F/78 Y | DLBCL | Gingiva | None | — | Biopsy | 3 W | — |
| 7 | Heibel et al. [34] | 2004 | –/70 Y | DLBCL | Oral mucosa | None | — | Biopsy | 1 M | 12 M |
| 8 | Sasaki et al. [35] | 2004 | F/54 Y | Primary cutaneous anaplastic large cell lymphoma | Forehead | None | — | Biopsy | 1 M | — |
| 9 | Chang et al. [36] | 2004 | F/40 Y | DLBCL | Orbit, conjunctiva | None | — | Biopsy | 5 W | 4 M |
| 10 | Winhoven et al. [37] | 2005 | M/39 Y | CD30 + anaplastic large cell T-cell lymphoma | Upper eyelid | Mometasone furoate (0.1% cream) | — | Biopsy | 2 M | 2 M |
| 11 | Sakuma et al. [38] | 2006 | F/70 Y | MALT-lymphoma | Hard palate | None | Sjögren syndrome | Biopsy | One month after first biopsy, histological confirmation 3 months after first biopsy | 38 M |
| 12 | Madan et al. [39] | 2007 | F/31 Y | Cutaneous peripheral T-cell lymphoma | Forehead | None | — | Biopsy | 2 M | 2 M |
| 13 | Ogden et al. [40] | 2008 | F/78 Y | B-cell lymphoma | Nose tip | Menthol 1% in aqueous cream and hydroxyzine 10 mg at night | — | Biopsy | — | — |
| 14 | Daly et al. [41] | 2008 | M/56 Y | T-cell lymphoma | Gingiva | None | T-cell lymphoma 14M earlier, different site | Biopsy | — | 4 Y |
| 15 | Graham et al. [16] | 2009 | M/82 Y | Primary cutaneous B-cell lymphoma, DLBCL | Left cheek, skin | None | — | Biopsy | — | — |
| 16 | Santiago-et-Sánchez-Mateos et al. [42] | 2011 | M/4 Y | Primary cutaneous anaplastic large cell lymphoma | Nose tip | None | — | Biopsy | 4 M | 4 M |
| 17 | Tamas et al. [43] | 2011 | F/66 Y | DLBCL with activated B-cell like immunophenotype | Between left vallecula and tongue | None | — | Biopsy | 6 M | 7 Y |
| 18 | Buckner et al. [44] | 2012 | F/67 Y | DLBCL | Maxillary sinus | None | Pneumonia and concomitant Clostridium Difficile-infection | Concomitant infection | — | 1 Y |
| 19 | Farquhar et al. [25] | 2014 | F/66 Y | Follicular B-cell lymphoma | Cheek | Steroids for unrelated asthma | Asthma | Steroids for unrelated asthma | 2 W | — |
| 20 | Igawa et al. [45] | 2015 | M/80 Y | Plasmablastic lymphoma | Gingiva | None | Epstein-Barr virus | Biopsy | 40 D | 5 M |
| 21 | Fernandez et al. [46] | 2015 | M/44 Y | T-cell lymphoma | Tip of the nose | None | — | Biopsy | 3 M | 1 Y |
| 22 | Kaibuchi et al. [47] | 2015 | M/87 Y | DLCBL | Gingiva | Azithromycin (prevention of infection after biopsy) | — | Biopsy | 3 W | 2.5 Y |
| 23 | Miyagawa et al. [48] | 2017 | M/46 Y | Primary cutaneous anaplastic large cell lymphoma | Upper lip | None | — | Biopsy | — | 14 M |
| 24 | Snijder et al. [49] | 2019 | F/88 Y | DLBCL | Cervical (level III) | None | Idiopathic pulmonary fibrosis | Biopsy | 3 M | 2 Y, 1 M |
| 25 | Pan et al. [50] | 2019 | F/55 Y | Small B cell lymphocytic cell lymphoma, IIIB | Neck, mediastinum, abdomen, inguinal | None | — | Biopsy on spleen | 4.5 Y | 4.5 Y |
| 26 | Flattow-Trujillo et al. [51] | 2019 | F/61 Y | DLBCL | Maxilla | None | Diabetes Mellitus | Biopsy | 6 W | 22 M |
| Present case | Peeters et al. | 2020 | M/59 Y | Follicular lymphoma | Buccal space to masticator space | None | — | Biopsy | 5.5 M | 6 M |
Summary of reported cases of spontaneous remission of non-Hodgkin lymphoma in the extracranial, head and neck area.
F, female; M, male; DLCBL, Diffuse Large B-Cell Lymphoma; MALT, Mucosa-associated lymphoid tissue; W, week; M, month; Y, year
In follicular lymphoma, the increase of follicular helper T-cells generates a contradictory immunosuppressive tumor microenvironment, thus promoting immune escape, tumor survival and growth [14, 24]. However in this case a large number of TFH cells were associated with a spontaneous regression after 6 months. Interestingly, one may raise the question whether the particularly favorable short-term outcome in this case could be linked to the brisk clonal TFH response, albeit there is no direct proof of it.
The finding of spontaneous remission avoided unnecessary treatment, highlighting the importance of clinical alertness in follicular lymphoma. As literature lacks statistical data regarding relapse after spontaneous remission of lymphoma and shows cases of relapse after spontaneous remission, close follow-up is strongly recommended. Moreover, the rare nature of the regression commands caution in interpreting this case report; postponing treatment or deviating from general treatment principles should be avoided.
Conclusion
It is important for the clinician, pathologist, radiologist and hematologist to be aware of the non-specific presentation of lymphoma. Mimicking other malign and benign lesions, lymphoma should be considered in the differential diagnosis when a tumor mass presents in the head and neck region. Moreover, the importance of clinical alertness is highlighted in this case, as a spontaneous remission was found unexpectedly, avoiding unnecessary treatment and implying close follow-up.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by EC Research UZ Leuven, University Hospitals Leuven; reference S64066. The patients/participants provided their written informed consent to participate in this study.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors met the requirements for authorship. MP wrote the manuscript with the support of JG, FC, LL, LM, TT, PV, and CP. JG, FC, LL, LM, TT, PV, and CP edited the manuscript. CP supervised the project. All authors provided critical feedback, and helped shape the research and manuscript.
Acknowledgments
We would like to express our appreciation to doctor K. Op de beeck (Radiology Department of the University Hospitals Leuven) for her radiologic assessment, and doctor B. Bisig (Pathology Department of the Lausanne University Hospital (CHUV) for her histological assessment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2021.642433/full#supplementary-material.
References
1.
Luminari S Bellei M Biasoli I Federico M Follicular lymphoma. Rev Bras Hematol Hemoter (2011) 34:54. 10.5581/1516-8484.20120015
2.
Jessy T . Immunity over inability: the spontaneous regression of cancer. J Nat Sci Biol Med (2011) 2:43. 10.4103/0976-9668.82318
3.
Vega F Lin P Medeiros LJ . Extranodal lymphomas of the head and neck. Ann Diagn Pathol (2005) 9:340. 10.1016/j.anndiagpath.2005.09.020
4.
Malaguarnera M Giordano M Russo C Puzzo L Trainiti M Consoli AS et al Lymphoma of cheek: a case report. Eur Rev Med Pharmacol Sci (2012) 16 Suppl 4(4 Suppl. l):4–7. PMID: 23090795.
5.
Gleeson MJ Bennett MH Cawson RA . Lymphomas of salivary glands. Cancer (1986) 58:699. 10.1002/1097-0142(19860801)58:3<699::AID-CNCR2820580317>3.0.CO;699-E
6.
Wright DH . Pathology of extra-nodal non hodgkin lymphomas. Clin Oncol (2012) 24:319. 10.1016/j.clon.2012.02.004
7.
Goodlad JR MacPherson S Jackson R Batstone P White J . Extranodal follicular lymphoma: a clinicopathological and genetic analysis of 15 cases arising at non-cutaneous extranodal sites. Histopathology (2004) 44:268. 10.1111/j.1365-2559.2004.01804.x
8.
Tart RP Mukherji SK Avino AJ Stringer SP Mancuso AA . Facial lymph nodes: normal and abnormal CT appearance. Radiology (1993) 13:35. 10.1148/radiology.188.3.8351335
9.
Pan W-R Le Roux CM Briggs CA . Variations in the lymphatic drainage pattern of the head and neck: further anatomic studies and clinical implications. Plast Reconstr Surg (2011) 127:611. 10.1097/PRS.0b013e3181fed511
10.
Bou-Assaly W . The forgotten lymph nodes: review of the superficial head and neck lymphatic system. J Radiol Imaging (2016) 19:172. 10.14312/2399-8172.2016-3
11.
Kukreti V Petersen P Pintilie M Tsang R Crump M Gospodarowicz M . Extranodal follicular lymphoma - a retrospective review and comparison with localized nodal follicular lymphoma. Blood (2004) 104:1375. 10.1182/blood.V104.11.1375.1375
12.
Buchpiguel CA . Current status of PET/CT in the diagnosis and follow up of lymphomas. Rev Bras Hematol Hemoter (2011) 33:140. 10.5581/1516-8484.20110035
13.
Johnson SA Kumar A Matasar MJ Schöder H Rademaker J . Imaging for staging and response assessment in lymphoma. Radiology (2015) 276:323. 10.1148/radiol.2015142088
14.
Carbone A Roulland S Gloghini A Younes A von Keudell G López-Guillermo A et al Follicular lymphoma. Nat Rev Dis Primers (2019) 5:132. 10.1038/s41572-019-0132-x
15.
Costa F Cian R Robiony M Zerman N Politi M . Unilateral swelling of the cheek. J Oral Maxillofac Surg (2008) 66:342. 10.1016/j.joms.2007.04.030
16.
Graham RM Thomson EF Cousin GC Kumar S Awasthi A . A case of facial lymphoma mimicking dental infection. Dental Update (2009) 36:244. 10.12968/denu.2009.36.4.244
17.
Weiler-Sagie M Bushelev O Epelbaum R Dann EJ Haim N Avivi I et al 18F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med (2010) 51:25. 10.2967/jnumed.109.067892
18.
Luminari S Biasoli I Arcaini L Versari A Rusconi C Merli F et al The use of FDG-PET in the initial staging of 142 patients with follicular lymphoma: a retrospective study from the FOLL05 randomized trial of the Fondazione Italiana Linfomi. Ann Oncol (2013) 24:2108. 10.1093/annonc/mdt137
19.
Bachy E Maurer MJ Habermann TM Gelas-Dore B Maucort-Boulch D Estell JA et al A simplified scoring system in de novo follicular lymphoma treated initially with immunochemotherapy. Blood (2018) 132(1):49–58. 10.1182/blood-2017-11-816405
20.
Federico M Bellei M Marcheselli L Luminari S Lopez-Guillermo A Vitolo U et al Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. Jco (2009) 27:4555. 10.1200/JCO.2008.21.3991
21.
Potts DA Fromm JR Gopal AK Cassaday RD . Spontaneous remission of an untreated, myc and BCL2 coexpressing, high-grade B-cell lymphoma: a case report and literature review. Case Rep Hematol (2017) 2017:1. 10.1155/2017/2676254
22.
Papac RJ . Spontaneous regression of cancer: possible mechanisms. In Vivo (Brooklyn) (1998) 12(6):571-8.
23.
Kumamoto M Nakamine H Hara T Yokoya Y Kawai J Ito H et al Spontaneous complete regression of high grade non-Hodgkin's lymphoma. Morphologic, immunohistochemical, and gene amplification analyses. Cancer (1994) 74:320. 10.1002/1097-0142(19941201)74:11<3023::AID-CNCR2820741120>3.0.CO;3023-Y
24.
Rawal S Chu F Zhang M Park HJ Nattamai D Kannan S et al Cross talk between follicular Th cells and tumor cells in human follicular lymphoma promotes immune evasion in the tumor microenvironment. J Immunol (2013) 190:6681. 10.4049/jimmunol.1201363
25.
Farquhar D Sobanko J Williams K Newman JG . A vanishing lymphoma in the cheek. ORL (2014) 76:189. 10.1159/000365853
26.
Hardin C . Unexpected diagnosis of follicular lymphoma during Mohs micrographic surgery. J Am Acad Dermatol (2018) 5:12. 10.1016/j.jaad.2018.05.1208
27.
Dhadlie S Strekozov B . Cutaneous extra nodal lymphoma relapse: a case report and review of literature. Int J Surg Case Rep (2018) 51:306. 10.1016/j.ijscr.2018.09.020
28.
Thaker R Lee KC Peters S Greenman D Kings JR . Asymptomatic nodule in the right cheek in a 65-year-old female. Oral Surg Oral Med Oral Pathol Oral Radiol (2019) 128:567. 10.1016/j.oooo.2019.02.017
29.
Burkitt DP Kyalwazi SK . Spontaneous remission of African lymphoma. Br J Cancer (1967) 21:14. 10.1038/bjc.1967.2
30.
Grem JL Hafez GR Brandenburg JH Carbone PP . Spontaneous remission in diffuse large cell lymphoma. Cancer (1986) 28:1097. 10.1002/1097-0142(19860515)57:10<2042::AID-CNCR2820571027>3.0.CO;2-#
31.
Poppema S Postma L Brinker M De Jong B . Spontaneous regression of a small non-cleaved cell malignant lymphoma (non-Burkitt's lymphoblastic lymphoma). Morphologic, immunohistological, and immunoglobulin gene analysis. Cancer (1988) 62:791. 10.1002/1097-0142(19880815)62:4<791::AID-CNCR2820620425>3.0.CO;791-M
32.
Savarrio L Gibson J Dunlop DJ O'Rourke N Fitzsimons EJ . Spontaneous regression of an anaplastic large cell lymphoma in the oral cavity: first reported case and review of the literature. Oral Oncol (1999) 35:609. 10.1016/S1368-8375(99)00034-2
33.
Koga M Kusukawa J Hayabuchi N . Spontaneous regression of extranodal malignant lymphoma occurred in the gingiva. Oral Oncol (2003) 39:323. 10.1016/S1368-8375(02)00122-7
34.
Heibel H Knödgen R Bredenfeld H Wickenhauser C Scheer M Zöller JE . Complete spontaneous remission of an aggressive non-hodgkin's lymphoma with primary manifestation in the oral cavity. Leuk Lymphoma (2004) 45:171. 10.1080/1042819031000139747
35.
Sasaki K Sugaya M Fujita H Takeuchi K Torii H Asahina A et al A case of primary cutaneous anaplastic large cell lymphoma with variant anaplastic lymphoma kinase translocation. Br J Dermatol (2004) 150:1202. 10.1111/j.1365-2133.2004.05987.x
36.
Chang Y-C Chang C-H Liu Y-T Tsai K-B Liu T-C Lin Y-N . Spontaneous regression of a large-cell lymphoma in the conjunctiva and orbit. Ophthal Plast Reconstr Surg (2004) 20:461. 10.1097/01.IOP.0000144791.05993.64
37.
Winhoven SM Murugesan S Coulson IH . Solitary CD30+ anaplastic large cell lymphoma of the eyelid showing regression. Br J Ophthalmol (2005) 89:385. 10.1136/bjo.2004.042804
38.
Sakuma H Okabe M Yokoi M Eimoto T Inagaki H . Spontaneous regression of intraoral mucosa-associated lymphoid tissue lymphoma: molecular study of a case. Pathol Int (2006) 56:331. 10.1111/j.1440-1827.2006.01967.x
39.
Madan V Cox NH . Primary cutaneous peripheral T-cell lymphoma, unspecified, that completely regressed after skin biopsy. Br J Dermatol (2007) 156:785. 10.1111/j.1365-2133.2007.07751.x
40.
Ogden S Coulson IH . B-cell lymphoma mimicking rhinophyma. Clin Exp Dermatol (2008) 33:213. 10.1111/j.1365-2230.2007.02476.x
41.
Daly R-M Healy CM Toner ME Flint SR . Spontaneous regression of non-Hodgkin's lymphoma in the oral cavity after incisional biopsy. Br J Oral Maxillofac Surg (2008) 46:223. 10.1016/j.bjoms.2007.03.010
42.
Santiago-et-Sánchez-Mateos D Hernández-Martín A Colmenero I Mediero IG León A Torrelo A . Primary cutaneous anaplastic large cell lymphoma of the nasal tip in a child. Pediatr Dermatol (2011) 28(5):570–5. 10.1111/j.1525-1470.2010.01245.x
43.
Tamás L Sári E Répássy G Szabó P Bagdi E Krenács L et al Spontaneous remission in localized diffuse large b-cell lymphoma. Pathol Oncol Res (2011) 17:779. 10.1007/s12253-011-9379-6
44.
Buckner TW Dunphy C Fedoriw YD van Deventer HW Foster MC Richards KL et al Complete spontaneous remission of diffuse large B-cell lymphoma of the maxillary sinus after concurrent infections. Clin Lymphoma Myeloma Leuk (2012) 12:455. 10.1016/j.clml.2012.06.007
45.
Igawa T Sato Y Kawai H Kondo E Takeuchi M Miyata-Takata T et al Spontaneous regression of plasmablastic lymphoma in an elderly human immunodeficiency virus (HIV)-negative patient. Diagn Pathol (2015) 10:421. 10.1186/s13000-015-0421-y
46.
González Fernández D Valdés Pineda F Gómez Díez S Vivanco Allende B . Primary cutaneous CD4+ small/medium-sized T-cell lymphoma with spontaneous regression after biopsy. Actas Dermo-Sifiliográficas (English Edition) (2015) 106:767. 10.1016/j.adengl.2015.09.015
47.
Kaibuchi N Okamoto T Kataoka T Kumasaka A Ando T . A case of spontaneous regression of lymphoma in the mandibular gingiva after biopsy. Oral Maxillofac Surg Cases (2015) 1:33. 10.1016/j.omsc.2015.06.002
48.
Miyagawa F Ogawa K Asada H . A case of CD4+/CD8+ double-positive primary cutaneous anaplastic large cell lymphoma of the lip involving spontaneous regression after biopsy. Eur J Dermatol (2017) 27:68. 10.1684/ejd.2016.2899
49.
Snijder J Mihyawi N Frolov A Ewton A Rivero G . Spontaneous remission in diffuse large cell lymphoma: a case report. J Med Case Rep (2019) 13. 10.1186/s13256-018-1937-z
50.
Pan Q Luo Y Cao X Zhang Y Li F . Spontaneous regression of clinically indolent lymphomas revealed by 18F-FDG PET/CT. Clin Nucl Med (2019) 44:321. 10.1097/RLU.0000000000002457
51.
Flatow-Trujillo L Win K Jencks A Andritsos L Arana Yi C . Spontaneous resolution of untreated diffuse large B-cell lymphoma of maxillary bone after incisional biopsy. Clin Case Rep (2019) 12:305. 10.1002/ccr3.2408
Appendix 1: PDF VECTRA
3D-view of the patient at the moment of first presentation, using VECTRA® H1 (Canfield Scientific Europe, Utrecht, Netherlands) (Supplementary Data Sheet 1). The swelling presented at the infraorbital region (it is observed best in cranial or caudal view). The zone of hypo-aesthesia is demarcated.
Figure
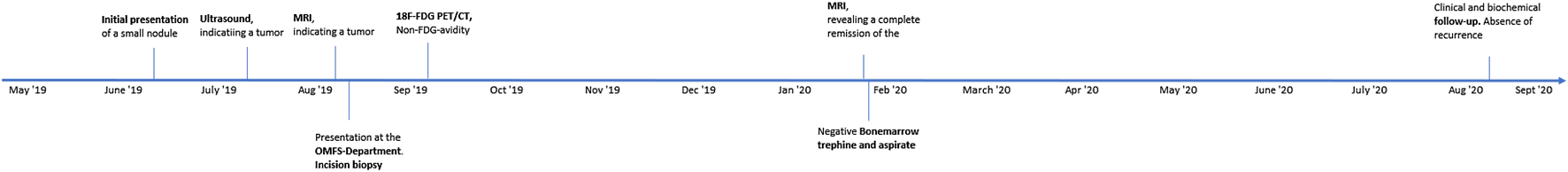
Appendix 2: Patient Timeline
MRI, Magnetic Resonance Imaging; OMFS-Department, Oral and Maxillofacial Surgery Department; 18F-FDG PET/CT, 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography.
Summary
Keywords
follicular lymphoma, cheek, spontaneous remission, head and neck neoplasms, case reports
Citation
Peeters M, Geusens J, Van der Cruyssen F, Michaux L, de Leval L, Tousseyn T, Vandenberghe P and Politis C (2021) Case Report: Spontaneous Remission of an Infraorbital Follicular B-Cell Lymphoma: Case Report and Review of the Literature. Pathol. Oncol. Res. 27:642433. doi: 10.3389/pore.2021.642433
Received
16 December 2020
Accepted
01 March 2021
Published
08 April 2021
Volume
27 - 2021
Edited by
Anna Sebestyén, Semmelweis University, Hungary
Updates
Copyright
© 2021 Peeters, Geusens, Van der Cruyssen, Michaux, de Leval, Tousseyn, Vandenberghe and Politis.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maxime Peeters, maxime.peeters.mp1@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.