Abstract
Background: Colorectal cancer (CRC) is the third most common type of cancer, it is considered a genetically heterogeneous disease with different molecular pathways being involved in its initiation and progression. Testes-specific protease 50 (TSP50) gene is a member of cancer/testis antigens that encodes for threonine protease enzyme. Overexpression of TSP50 was found to enhance the progression and invasion of breast cancer and other malignant tumors. SERCA2 is widely expressed in several body tissues; its aberrant expression has been involved in many cancers. IL-8 is an inflammatory cytokine. Alongside its role in inflammation, its expression was reported to induce the migration of tumor cells.
Aim: Study the expression of TSP50, SERCA2 and IL-8 in colorectal adenoma (CRA), CRC and normal colonic tissues to compare the expression of these biomarkers in relation to clinicopathological parameters and prognostic factors.
Results: TSP50, SERCA2 and IL-8 expression varied between normal colonic tissues, CRA and CRC. Significant statistical association was detected between the three biomarkers’ overexpression and degree of dysplasia in CRA. Also, significant statistical relation was found between the three biomarkers’ overexpression and presence of lympho-vascular invasion, advanced TNM staging and high intra-tumoral inflammatory infiltrate. Multivariable analysis showed that the overexpression of the three biomarkers is significantly associated with worse prognosis.
Conclusion: The expression of TSP50, SERCA2 and IL-8 was different between the normal tissue and neoplastic colorectal tissue on one hand and between CRA and CRC on the other. Increased expression of these biomarkers in neoplastic epithelial cells of colorectal carcinoma is associated with adverse prognostic factors and could be considered as independent prognostic factors.
Introduction
Colorectal cancer (CRC) is the third most common type of cancer, with a worldwide incidence of about 24.8% in 2020. In Egypt; CRC ranked tenth in males (5.8%) and ninth in females (6.2%) [1, 2]. It accounts for about 50% of all gastrointestinal tract-cancers with adenocarcinoma being the predominant histopathological type. Despite the continuous improvement in diagnosis and therapy, approximately 60% of colon cancer patients develop metastases [3].
Colorectal cancer is a genetically heterogeneous disease in which several and different molecular pathways are involved in transformation of normal colonic epithelium to malignant cells, tumor proliferation and invasion. The expanding knowledge of the molecular pathology of colon cancer resulted in classification of CRC into characteristic molecular subtypes based on gene expression in both tumor cells and infiltrating non tumorous stromal cells, to increase the accuracy of prognosis and design personalized therapies for better management of patients [4].
Testes-specific protease 50 (TSP50) gene, a member of cancer/testis antigens, encodes for a threonine protease enzyme which was first discovered in breast cancer cells. It is expressed in the normal spermatocytes of testes and is related to spermatogenesis [5]. Abnormal activation and overexpression of TSP50 was found to enhance the progression and invasion of breast cancer and other malignant tumors such as gastric [6], cervical [7] and lung [8] cancers. Recent studies reported that activation of TSP50 gene through DNA hypomethylation stimulates proliferation and transformation of cells while hypermethylation with subsequent TSP50 gene silencing or knocking down are associated with inhibition of cell proliferation, migration and inducing apoptosis [7]. As hypomethylation is common and prominent in CRC compared to normal colorectal tissue, TSP50 could be considered as a potential oncogene. In addition, TSP50 gene locus; 3p21.31 has been recognized as a susceptible locus for CRC in the Chinese population [9].
Disruption of calcium homeostasis becomes evident as an important step in the processes of tumorigenesis and metastasis through upregulation of genes’ expression which are involved in cellular proliferation, angiogenesis and invasion [10]. The level of intracellular Ca2+ is tightly regulated by Ca2+ channels and Ca2+ pumps which control the movement of Ca2+ across the plasma membrane and intracellular storage organelles [11]. One of the Ca2+ pumps belonging to the family of P-Type ATPases is Sarco/Endoplasmic Reticulum Ca2+-ATPase or SERCA. SERCA is responsible for segregating calcium in the endoplasmic reticulum (ER), the richest store for intracellular calcium [12]. There are 3 genes coding the SERCA1- 3 pumps, with different expression levels and tissue distributions in the body, one of these three isoforms is SERCA2 which is widely expressed in several body tissues [13]. Aberrant expression of SERCA2 either up-or downregulation have been involved in many cancers such as lung and prostatic cancers mirroring the link between disturbances in Ca2+ homeostasis on one hand and cancer initiation and progression on the other hand [14]. However the exact role of SERCA2 expression in CRC and its relation to clinicopathological factors have not been cleared up.
Colorectal carcinoma has been found to express a variety of chemokines, including the multifunctional cytokine interleukin-8 (IL-8), a member of the CXC chemokine family of inflammatory cytokines that stimulates the migration of distinct subsets of cells [15]. IL-8 and other inflammatory cytokines have been shown to act as potent chemo-attractants for leucocytes, such as neutrophils and natural killer cells [16]. In recent years, IL-8 was reported to induce the migration of tumor cells and its expression was correlated with tumor growth, angiogenesis and metastatic potential in various human carcinomas [17-19].
The expression of TSP50 as a potential oncoprotein in association with SERCA2 and inflammatory cytokine (IL-8) in colorectal adenoma (CRA) and CRC and the relation of these biomarkers’ expression to intratumoral inflammatory infiltrate and other clinicopathological and prognostic factors in CRC have not been clarified. Thus, the aim of this work is to study the expression of TSP50, SERCA2 and IL-8 in CRA and CRC to find the relation of these biomarkers’ expression to intratumoral inflammatory infiltrate and other clinicopathological and prognostic factors.
Materials and Methods
After the approval of local ethical committee, Faculty of Medicine, Tanta University (34340/12/20), 107 selected specimens of CRA and primary CRC (29 CRA and 78 CRC) along with 12 normal colonic mucosal tissues obtained from non-specific colonic lesions, were retrospectively collected from the archive of pathology department and private labs. The specimens were either colonoscopic biopsies (for CRA) or colectomies (for CRC). None of colorectal carcinoma cases had received neoadjuvant chemotherapy.
H&E stained sections were revised to confirm the diagnosis and to assess the grade of dysplasia of CRA, the degree of tumor differentiation, lympho-vascular invasion (LVI) and the intra-tumoral inflammatory cell infiltrate score of CRC. The dysplasia of CRA was graded according to a two-tier grading system into a low and high-grade dysplasia (LGD and HGD respectively) [20]. Colorectal carcinomas were graded into three grades; well, moderately and poorly differentiated [21] and they were staged according to TNM staging system [22]. The intra-tumoral inflammatory cellular infiltrate [lymphocytes (ITL) and neutrophils (ITN)] of CRC was evaluated and scored as follow: score 0; no increase in inflammatory cells, score 1; a mild increase in inflammatory cells without destruction of the cancer cells, score 2; a moderate increase in inflammatory cells with some destruction of the cancer cells, and score 3; severe inflammatory reaction with frequent destruction of the cancer cell islet. Specimens were further grouped into either low-inflammatory group and high-inflammatory group. Tumors with scores 0 and 1 were categorized as low-inflammatory, while those with scores 2 and 3 were categorized as high-inflammatory group [23].
For immunohistochemistry, paraffin blocks of embedded tissues were cut into 5-μm-thick sections, deparaffinized in two changes xylene, rehydrated in two changes of alcohol then rinsed in distilled water. Antigen retrieval was performed by first preheating the staining dish containing citrate buffer (10 mM citric acid, 0.05% Tween20, pH 6.0) until temperature reaches 95–100°C then sections were incubated in it for 20–40 min. Sections were incubated overnight at 4°C, to increase the affinity of the antibodies used to their antigens, with the following primary antibodies: anti-TSP50 polyclonal (1:25; ABclonal, United States), anti-SERCA2 polyoclonal (1:100; ABclonal, United States) and anti-IL-8 polyclonal (1:100; ABclonal, United States). The slides were then counter stained with hematoxylin. Breast carcinoma was used as a positive control for TSP50. For IL-8 and SERCA2, infiltrating neutrophils were used as an internal positive control.
Immunohistochemical Assessment
Immunohistochemical evaluation of TSP50 protein cytoplasmic staining was performed using semi-quantitative scoring method by multiplying the intensity of staining and the percentage of positive tumor cells. For staining intensity, the sections were scored as 0 (negative), 1 (mild), 2 (moderate) and 3 (strong), and for the percentages: 0 (0–10%), 1 (10–30%), 2 (31–50%), 3 (51–70%) and 4 (71–100%). The final scores were graded as follows: − (0); + (1–3); ++ (4–8); +++ (9–12). The sections were classified into two groups: low expression (−,+); and high expression (++,+++) for statistical analysis [24].
For SERCA2, the specimens were considered positive if >5% of the colonic epithelial cells showed cytoplasmic and/or plasma membrane staining. Semiquantitative scores were used for SERCA2 stains according to the percentage of positively stained cells (+1: <25%; +2: 25–50%; +3: 51–75%; and +4: >75%). Tumors showing expression scores of ++, +++, or ++++ were considered as the high expression group, whereas those with scores of + or negative staining were regarded as the low expression group [25].
Cytoplasmic and membranous staining for IL-8 were evaluated. The intensity of staining was scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). The percentage of stained cells was grouped as: no staining = 0, 1–10% of stained cells = 1, 11–50% = 2, 51–80% = 3, and 81–100% = 4. Values of <4 and ≥4 divide the patients into low and high IL-8 expression groups respectively [26].
Statistical Analysis
Statistical analysis was conducted using SPSS (version 20) (Chicago, IL, United States). The association between clinicopathological factors and the expression of biomarkers was performed using Chi‐square (x2) test. Correlation between biomarkers’ expression was performed using Spearman correlation (r) and regression analysis was used for multivariable analysis of prognostic factors. The results were considered as statistically significant if the p value was <0.05.
Results
Clinicopathological Characteristics of CRA Cases
For the 29 CRA cases, the age of patients ranged from 40–62 years with a mean age of (42.3 ± 2.08). Eighteen (62.1%) cases were males and the other eleven (37.9%) cases were females. Twenty four adenomas were located in the colon (16 in the left side and 8 in the right side) and five adenomas (17.2%) in the rectum. Thirteen (44.8%) adenomas were of tubular type and the remaining 16 (55.2%) showed tubulovillous configuration. LGD was detected in 15 (51.7%) and HGD in 14 (48.3%) CRA cases Table 1.
TABLE 1
| Factor | CRA n (%) |
|---|---|
| Age | |
| Mean ± SD | 42.3 ± 2.08 |
| <45 | 6 (20.7) |
| ≥45 | 23 (79.3) |
| Sex | |
| Male | 18 (62.1) |
| Female | 11 (37.9) |
| Anatomical site | |
| Colon | |
| Right side | 8 (27.6) |
| Left side | 16 (55.2) |
| Rectum | 5 (17.2) |
| Histological type | |
| Tubular | 13 (44.8) |
| Tubulovillous | 16 (55.2) |
| Dysplasia | |
| LGD | 15 (51.7) |
| HGD | 14 (48.3) |
Clinicopathologic characteristics of CRA cases.
LGD, low-grade dysplasia; HGD, high-grade dysplasia.
Clinicopathological Characteristics of CRC Cases
A total of 78 CRC cases were studied, the age of patients was ranged from 42–72 years with a mean age of (60.4 ± 7.41), 48 (61.5%) cases were males and 30 (38.5%) cases were females. Twenty (25.6%) tumors were located in the right colon, 46 (59%) tumors were in the left colon and 12 (15.4%) tumors were in the rectum. Most of the cases were adenocarcinoma NOS type (64.1%). Moderate and poor degrees of differentiation were detected in 38 (48.7%) and 22 (28.2%) carcinomas respectively, while well differentiated carcinomas were found in 18 (23.1%) specimens. LVI was found in 45 tumors (57.7%). For TNM staging, T1 tumors were identified in 11 (14.1%) cases, T2 in 53 (68%) cases and T3 in 14 (17.9%) cases. The regional lymph node invasion was recognized in 65 cases (83.3%) and distant metastasis was found in 30 (38.5%) cases. For intra-tumoral inflammation, high ITL and ITN scores were encountered in 40 (51.3%) and 38 (48.7%) tumors respectively Table 2.
TABLE 2
| Clinicopathologic characteristics | n (%) |
|---|---|
| Age | |
| Mean ± SD | 60.4 ± 7.41 |
| <45 | 18 (23.1) |
| ≥45 | 60 (76.9) |
| Sex | |
| Male | 48 (61.5) |
| Female | 30 (38.5) |
| Anatomical site | |
| Colon | |
| Right side | 20 (25.6) |
| Left side | 46 (59) |
| Rectum | 12 (15.4) |
| Histological type | |
| Adenocarcinoma NOS | 50 (64.1) |
| Mucinous | 18 (23.1) |
| Signet ring | 10 (12.8) |
| Grade | |
| I | 18 (23.1) |
| II | 38 (48.7) |
| III | 22 (28.2) |
| LVI | |
| Absent | 33 (42.3) |
| Present | 45 (57.7) |
| TNM Staging | |
| T | |
| T1 | 11 (14.1) |
| T2 | 53 (68) |
| T3 | 14 (17.9) |
| N | |
| N0 | 13 (16.7) |
| N1 | 49 (62.8) |
| N2 | 16 (20.5) |
| M | |
| M0 | 48 (61.5) |
| M1 | 30 (38.5) |
| ITL | |
| Low | 34 (43.6) |
| High | 44 (56.4) |
| ITN | |
| Low | 40 (51.3) |
| High | 38 (48.7) |
Clinicopathological characteristics of CRC cases.
LVI, lympho-vascular invasion; T, primary tumor size; N, regional lymph node spread; M, distant metastasis; ITL, intra-tumoral lymphocytes; ITN, intra-tumoral neutrophils.
Expression of TSP50, SERCA2 and IL-8 in Neoplastic Cells of CRA
High TSP50 expression was observed in 15 adenomas and it was significantly identified more in CRAs with tubulovillous architecture (68.8%) compared to tubular ones (30.8%) and in adenomas HGD (71.4%) than adenomas with LGD (33.3%) (p = 0.042 and 0.040 respectively). No significant statistical relation was found between TSP50 immunostaining and CRA location (p = 0.564) Table 3 and Figures 1B,C.
TABLE 3
| Factor | TSP50 | SERCA2 | IL-8 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low n = 14 | High n = 15 | x 2 /p value | Low n = 16 | High n = 13 | x 2 /p value | Low n = 19 | High n = 10 | x 2 /p value | |
| Histological type | |||||||||
| Tubular | 9 (69.2) | 4 (30.8) | 4.144/0.042* | 8 (61.5) | 5 (38.5) | 0.388/0.534 | 10 (77) | 3 (23) | 1.357/0.244 |
| Tubulovillous | 5 (31.3) | 11 (68.8) | 8 (50) | 8 (50) | 9 (56.3) | 7 (43.7) | |||
| Location | |||||||||
| Colonic | 11 (45.8) | 13 (54.2) | 0.333/0.564 | 12 (50) | 12 (50) | 1.506/0.220 | 15 (62.5) | 9 (37.5) | 0.561/0.454 |
| Rectal | 3 (60) | 2 (40) | 4 (80) | 1 (20) | 4 (80) | 1 (20) | |||
| Dysplasia | |||||||||
| LGD | 10 (66.7) | 5 (33.3) | 4.209/0.040* | 11 (73.3) | 4 (26.7) | 4.144 | 13 (86.7) | 2 (13.3) | 6.152/0.013* |
| HGD | 4 (28.6) | 10 (71.4) | 5 (35.7) | 9 (64.3) | 0.042* | 6 (42.9) | 8 (57.1) | ||
Expression of TSP50, SERCA2 and IL-8 in CRA
*p value < 0.05.
LGD, low grade dysplasia; HGD, high grade dysplasia; CRA, colorectal adenoma.
FIGURE 1
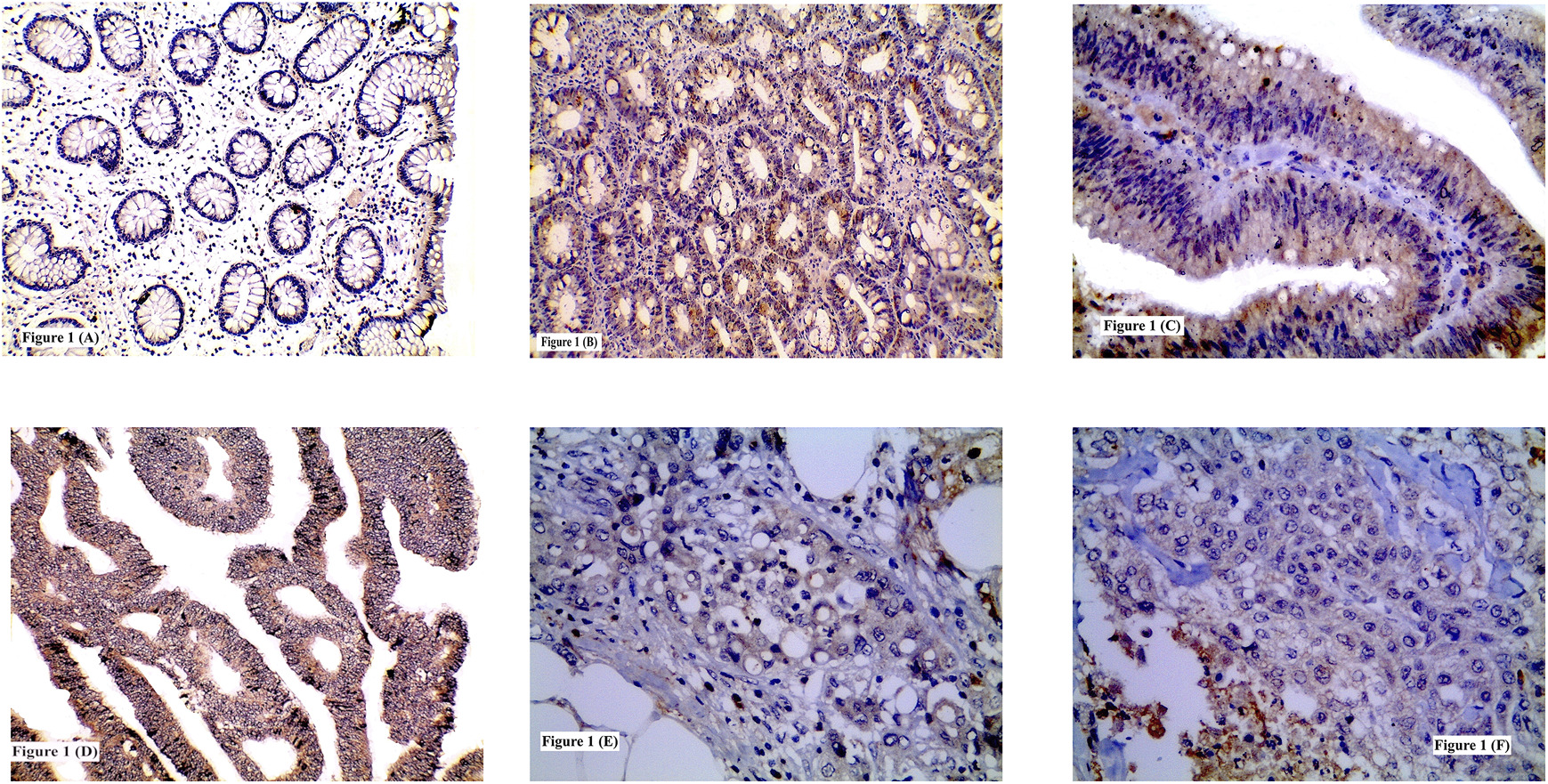
Immunohistochemical expression of TSP50 showing: (A) Low expression in normal colonic tissue; (B) High expression in tubular adenoma; (C) High expression in tubulovillous adenoma with high grade dysplasia; (D) High expression in grade II CRC; (E) High expression in signet ring CRC; (F) Low expression in grade III CRC. (A, B, D ×200) (C, E, F ×400).
Higher SERCA2 immunolabelling was detected in tubulovillous (50%) more than tubular adenomas (38.5%) and in CRAs with HGD more than those with LGD (64.3 and 26.7% respectively). Significant statistical relation was recognized between high SERCA2 expression and the degree of dysplasia (p = 0.042). No significant statistical difference was found between SERCA2 expression and CRA histologic type or location (p = 0.534 and 0.220 respectively) Table 3 and Figures 2A,B.
FIGURE 2
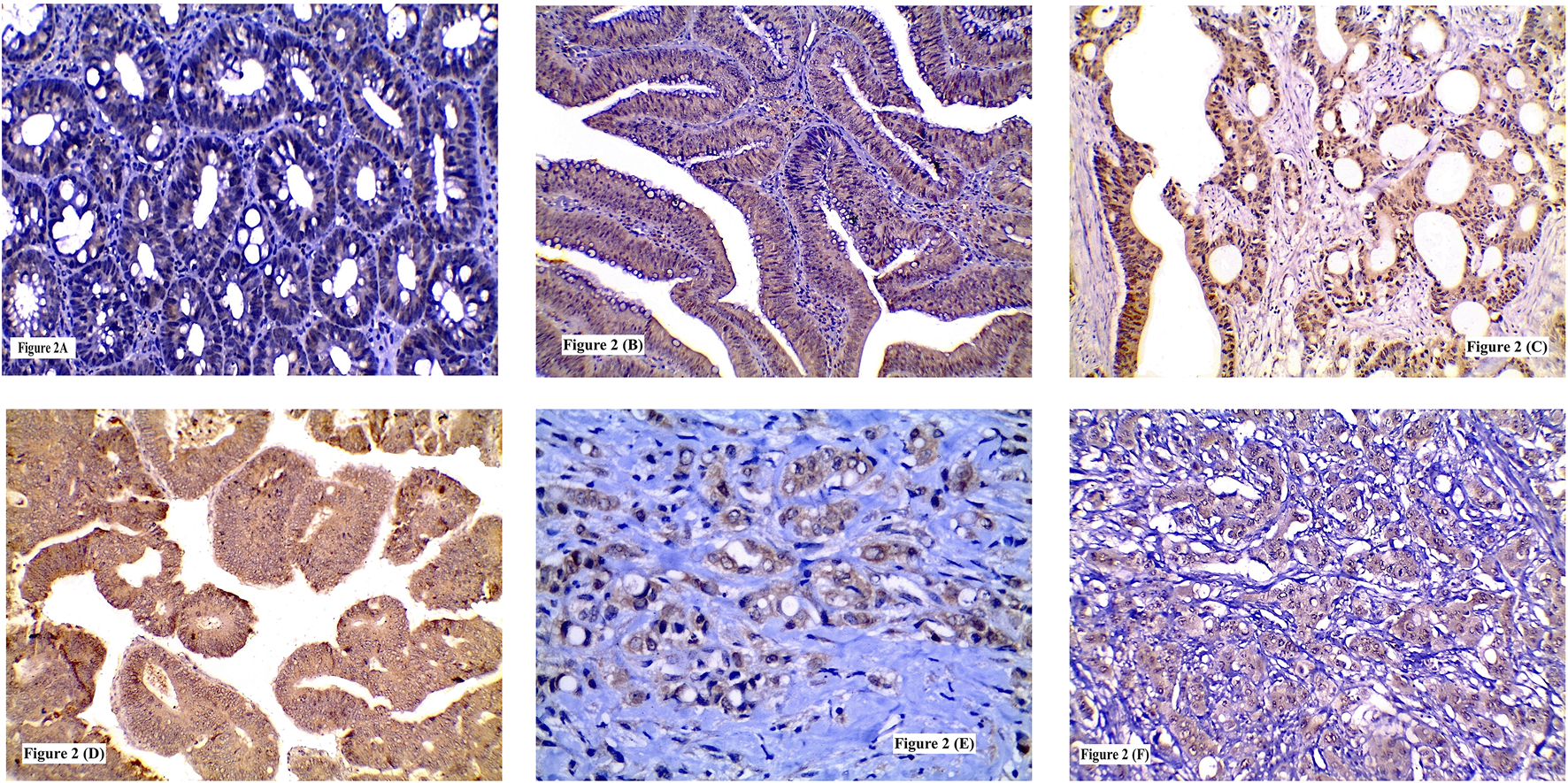
Immunohistochemical expression of SERCA2 showing: (A) Low expression in low grade tubular adenoma; (B) High expression in high grade tubulovillous adenoma; (C) High expression in grade I CRC; (D) High expression in grade II CRC; (E) High expression in signet ring CRC; (F) High expression in grade III CRC. (A, B, C, D, F ×200) (E ×400).
IL-8 high expression was recognized in 10 CRA whereas low expression was detected in 19 CRA. No significant statistical relation was found between IL-8 expression and adenoma type or location (p = 0.244 and 0.454 respectively). High IL-8 immunostaining was significantly detected more frequently in CRA with HGD (57.1%) than LGD (13.3%) (p = 0.013) Table 3 and Figures 3B,C.
FIGURE 3
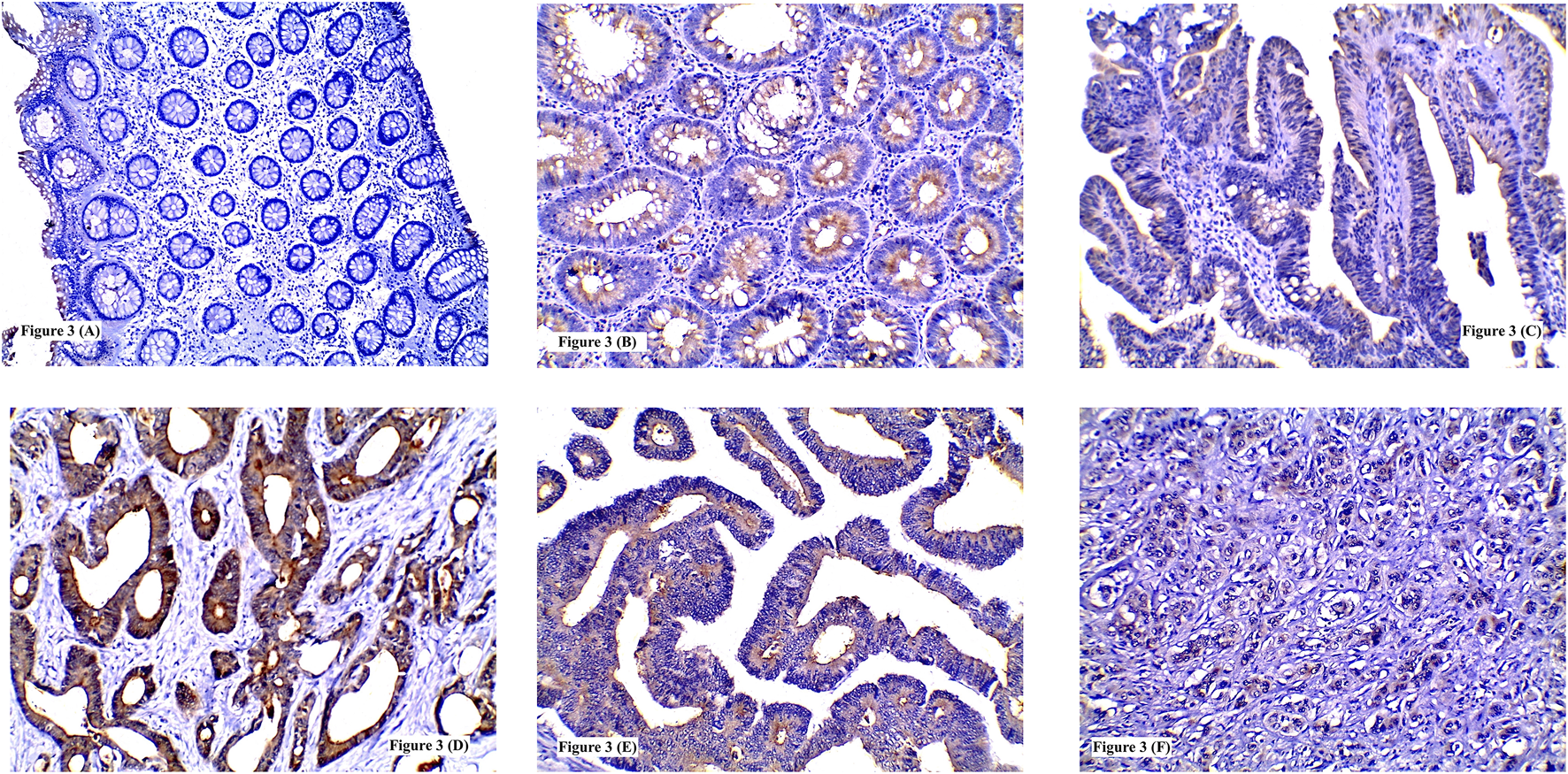
Immunohistochemical expression of IL-8 showing: (A) Low expression in normal colonic tissue; (B) High expression in tubular adenoma; (C) Low expression in tubulovillous adenoma with low grade dysplasia; (D) High expression in grade I CRC; (E) High expression in grade II CRC; (F) High expression in poorly differentiated CRC. (A, F ×100) (B, C, D, E ×200).
Expression of TSP50, SERCA2 and IL-8 in the Neoplastic Cells of CRC
High TSP50 expression was found in 47/78 cases and it was significantly associated with the presence of LVI (73.3%, p = 0.006), advanced TNM staging (T stage; p = 0.008, N stage; p = 0.0001 and M stage; p = 0.0001) and high intra-tumoral inflammatory scores whether lymphocytic (75%, p = 0.002) or neutrophilic (76.3%, p = 0.005). TSP50 protein overexpression was demonstrated in 55.6, 71 and 45.5% of grade I, II and III tumors respectively. Although poorly differentiated tumors showed high TSP50 expression less frequently than well and moderately differentiated tumors in neoplastic cells, however non-neoplastic stromal cells of grade III tumors exhibited high TSP50 protein expression. No statistically significant relationship was detected between TSP50 immunostaining and tumor location (p = 0.882), histological type (p = 0.073) or grade (p = 0.133) Table 4 and Figures 1D–F.
TABLE 4
| Factor | TSP50 | SERCA2 | IL-8 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low n = 31 n (%) | High n = 47 n (%) | x 2 /p value | Low n = 37 n (%) | High n = 41 n (%) | x 2 /p value | Low n = 34 n (%) | High n = 44 n (%) | x 2 /p value | |
| Anatomical site | |||||||||
| Colon | |||||||||
| Right side | 12 (60) | 8 (40) | 0.022/0.882 | 9 (45) | 11 (55) | 1.131/0.288 | 6 (30) | 14 (70) | 0.021/0.884 |
| Left side | 14 (30.4) | 32 (69.6) | 24 (52.2) | 22 (47.2) | 23 (50) | 23 (50) | |||
| Rectum | 5 (41.7) | 7 (58.3) | 4 (33.3) | 8 (66.7) | 5 (41.7) | 7 (58.3) | |||
| Histological type | |||||||||
| Adenocarcinoma NOS | 16 (32) | 34 (68) | 5.241/0.073 | 23 (46) | 27 (54) | 0.739/0.691 | 17 (34) | 33 (66) | 5.213/0.074 |
| Mucinous | 8 (44.4) | 10 (55.6) | 10 (55.6) | 8 (44.4) | 11 (61.1) | 7 (38.9) | |||
| Signet ring | 7 (70) | 3 (30) | 4 (40) | 6 (60) | 6 (60) | 4 (40) | |||
| Grade | |||||||||
| I | 8 (44.4) | 10 (55.6) | 4.028/0.133 | 13 (72.2) | 5 (27.7) | 8.022/0.018* | 14 (77.8) | 4 (22.2) | 11.253/0.004* |
| II | 11 (29) | 27 (71) | 18 (47.4) | 20 (52.6) | 12 (31.6) | 26 (68.4) | |||
| III | 12 (54.5) | 10 (45.5) | 6 (27.3) | 16 (72.7) | 8 (36.4) | 14 (63.6) | |||
| LVI | |||||||||
| Absent | 19 (57.6) | 14 (42.4) | 7.595/0.006* | 21 (63.6) | 12 (36.4) | 6.021/0.014* | 23 (69.7) | 10 (30.3) | 15.855/0.0001* |
| Present | 12 (26.7) | 33 (73.3) | 16 (35.6) | 29 (64.4) | 11 (24.4) | 34 (75.6) | |||
| TNM Staging | |||||||||
| T | |||||||||
| T1 | 9 (81.8) | 2 (18.2) | 9.601/0.008* | 10 (90.9) | 1 (9.1) | 12.234/0.002* | 8 (72.7) | 3 (27.3) | 8.749/0.013* |
| T2 | 18 (34) | 35 (66) | 24 (45.3) | 29 (54.7) | 24 (45.3) | 29 (54.7) | |||
| T3 | 4 (28.6) | 10 (71.4) | 3 (21.4) | 11 (78.6) | 2 (14.3) | 12 (85.7) | |||
| N | |||||||||
| N0 | 12 (92.3) | 1 (7.7) | 18.157/0.0001* | 10 (77) | 3 (23) | 9.860/0.007* | 11 (84.6) | 2 (15.4) | 11.609/0.003* |
| N1 | 15 (30.6) | 34 (69.4) | 24 (49) | 25 (51) | 19 (38.8) | 30 (61.2) | |||
| N2 | 4 (25) | 12 (75) | 3 (18.8) | 13 (81.2) | 4 (25) | 12 (75) | |||
| M | |||||||||
| M0 | 28 (58.3) | 20 (41.7) | 18.009/0.0001* | 30 (62.5) | 18 (37.5) | 11.358/0.001* | 26 (54.2) | 22 (45.8) | 5.678/0.017* |
| M1 | 3 (10) | 27 (90) | 7 (23.3) | 23 (76.7) | 8 (26.7) | 22 (73.3) | |||
| ITL | |||||||||
| Low | 20 (58.8) | 14 (41.2) | 9.162/0.002* | 19 (55.9) | 15 (44.1) | 1.725/0.189 | 22 (64.7) | 12 (35.3) | 10.930/0.001* |
| High | 11 (25) | 33 (75) | 18 (41) | 26 (59) | 12 (27.3) | 32 (72.7) | |||
| ITN | |||||||||
| Low | 22 (55) | 18 (45) | 7.908/0.005* | 29 (72.5) | 11 (27.5) | 20.686/0.0001* | 24 (60) | 16 (40) | 8.992/0.003* |
| High | 9 (23.7) | 29 (76.3) | 8 (21.1) | 30 (78.9) | 10 (26.3) | 28 (73.7) | |||
Expression of TSP50, SERCA2 and IL-8 in neoplastic cells of CRC.
*p value < 0.05.
LVI, lympho-vascular invasion; T, primary tumor size; N, regional lymph node spread; M, distant metastasis; ITL, intra-tumoral lymphocytes; ITN, intra-tumoral neutrophils.
SERCA2 cytoplasmic overexpression in neoplastic cells was identified in only 5 grade I tumors (27.7%), most of the grade II tumors (52.6%) and in the majority of grade III tumors (72.7%). High SERCA2 expression was found more frequently among tumors with LVI (64.4%) than those without (36.4%). Moreover, SERCA2 overexpression was linked with advanced TNM staging. Tumors with high ITN infiltration (78.9%) showed higher SERCA2 protein expression than those with low ITN infiltration (27.5%) or ITL (either low-or high, 44.1 and 59% respectively) infiltration. Significant statistical association was revealed between high SERCA2 protein expression and tumor grade (p = 0.018), LVI (p = 0.014), TNM staging (p = 0.002, 0.007 and 0.001 respectively) and high ITN infiltration (p = 0.0001). No significant relation was found between SERCA2 immunolabelling and tumor location (p = 0.288), histological type (p = 0.691) or ITL infiltration (p = 0.189) Table 4 and Figures 2C–F.
High IL-8 immunoreactivity was identified in 44/78 tumors. This high IL-5 protein expression was significantly detected in moderately differentiated (68.4%) and poorly differentiated (63.6%) carcinomas compared to well differentiated ones (22.2%) (p = 0.004). Also, tumors with LVI (75.6%) showed significant higher IL-8 expression more than those without LVI (30.3%) (p = 0.0001). Similarly, increased IL-8 expression was significantly linked to increase in tumor size (85.7%) (p = 0.013) advanced N staging (N2 = 75%) (p = 0.003) and presence of distant metastasis (73.3%) (p = 0.017). Tumors with high ITL and/or ITN exhibited significant high IL-8 protein immunolabeling (72.7 and 73.7% respectively) (p = 0.001 and 0.003 respectively). No significant statistical association was found between IL-8 immunoreactivity and tumor site (p = 0.884) or histological type (p = 0.074) Table 4 and Figures 3D–F.
Multivariable analysis of independent prognostic factors: anatomical site, histological type, tumor grade, LVI (absent versus present), TN staging, intra-tumoral inflammatory infiltrate (low versus high), expression of TSP50, SERCA2 and IL-8 proteins (low expression versus high) was conducted showing that TSP50, SERCA2 and IL-8 overexpression were significantly associated with adverse prognosis along with high tumor grade, LVI, advanced T stage, presence of regional lymph node invasion and high ITL Table 5.
TABLE 5
| Metastasis | Multivariable p value | ||
|---|---|---|---|
| Anatomical site | |||
| Colon | |||
| Right side | 7/20 | 35% | 0.451 |
| Left side | 17/46 | 36.9% | |
| Rectum | 6/12 | 50% | |
| Histological type | |||
| Adenocarcinoma NOS | 20/50 | 40% | 0.656 |
| Mucinous | 4/18 | 22.2% | |
| Signet ring | 6/10 | 60% | |
| Grade | |||
| I | 0/18 | 0% | <0.0001* |
| II | 18/38 | 47.4% | |
| III | 12/22 | 54.5% | |
| LVI | |||
| Absent | 6/33 | 18.2% | 0.001* |
| Present | 24/45 | 53.3% | |
| T | |||
| T1 | 0/11 | 0% | 0.004* |
| T2 | 22/53 | 41.5% | |
| T3 | 8/14 | 57.1% | |
| N | |||
| N0 | 0/13 | 0% | 0.025* |
| N1 | 23/49 | 46.9% | |
| N2 | 7/16 | 43.8% | |
| ITL | |||
| Low | 11/34 | 32.4% | 0.026* |
| High | 19/44 | 43.2 | |
| ITN | |||
| Low | 16/40 | 40% | 0.778 |
| High | 14/38 | 36.8% | |
| TSP50 | |||
| Low | 3/31 | 9.7% | <0.0001* |
| High | 27/47 | 51.1% | |
| SERCA2 | |||
| Low | 7/37 | 18.9% | 0.001* |
| High | 23/41 | 56.1% | |
| IL-8 | |||
| Low | 8/34 | 23.5% | 0.017* |
| High | 22/44 | 50% | |
Multivariable analysis of factors affecting prognosis in colorectal carcinoma cases.
*p value < 0.05.
LVI, lympho-vascular invasion; T, primary tumor size; N, regional lymph node spread; ITL, intra-tumoral lymphocytes; ITN, intra-tumoral neutrophils.
Correlation Between TSP50, SERCA2 and IL-8 Expression in the Neoplastic Cells of CRC
A significant positive correlation was found between TSP50 expression on one side and SERCA2 and IL-8 expression on the other side. As 32 (78%) and 37 (84.1%) tumors of high SERCA2 and IL-8 immunoreactivity respectively showed TSP50 overexpression as well (r = 0.383 and 0.554, p = 0.001 and <0.0001 respectively). Furthermore, A significant statistical positive correlation was detected between SERCA2 and IL-8 expression, in which 33 (75%) tumors with high IL-8 immunostaining showed high SERCA2 expression too (r = 0.511, p < 0.0001) Table 6.
TABLE 6
| TSP50 | r | p Value | SERCA2 | r | p Value | |||
|---|---|---|---|---|---|---|---|---|
| Low n (%) | High n (%) | Low n (%) | High n (%) | |||||
| SERCA2 | ||||||||
| Low | 22 (59.5) | 15 (40.5) | 0.383 | 0.001* | - | - | - | - |
| High | 9 (22) | 32 (78) | - | - | - | - | ||
| IL-8 | ||||||||
| Low | 24 (70.6) | 10 (29.4) | 0.554 | <0.0001* | 26 (76.4) | 8 (23.5) | 0.511 | <0.0001* |
| High | 7 (15.9) | 37 (84.1) | 11 (25) | 33 (75) | ||||
Correlation between TSP50, SERCA2 and IL-8 expression in the neoplastic cells of CRC.
*p value < 0.05.
r, correlation.
Comparing the Expression of TSP50, SERCA2 and IL-8 in Normal Tissues, CRA and CRC
TSP50 expression was variable, being low in colorectal normal epithelium (Figure 1A), low-to- high in CRA and mostly high in CRC. TSP50 expression scores in CRC were significantly higher than those in colorectal normal tissues or CRA (p = 0.001). SERCA2 immunoreactivity also varied between normal epithelium, CRA CRC cases. Significant increase in SERCA2 expression was observed in CRC (p = 0.003). Similarly, IL-8 protein was significantly higher in CRCs compared to normal epithelium specimens (Figure 3A) and CRA (p < 0.0001) Table 7.
TABLE 7
| Factor | TSP50 | SERCA2 | IL-8 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | x2/p value | Low | High | x2/p value | Low | High | x2/p value | |
| Normal | 12 (100) | 0 (0) | 15.133/0.001* | 12 (100) | 0 (0) | 11.598/0.003* | 12 (100) | 0 (0) | 15.718/<0.0001* |
| CRA | 14 (48.3) | 15 (51.7) | 16 (55.2) | 13 (44.8) | 19 (65.5) | 10 (34.5) | |||
| CRC | 31 (39.7) | 47 (60.3 | 37 (47.4) | 41 (52.6) | 34 (43.6) | 44 (56.4) | |||
Expression of TSP50, SERCA2 and IL-8 in normal tissues, CRA and CRC cases.
*p value < 0.05.
CRA, colorectal adenoma; CRC, colorectal carcinoma.
Discussion
Colorectal carcinoma still remains a challenging disease due to several contributing factors: a variety of molecular transformation pathways other than the traditional APC, KRAS and tumor suppressor gene p53 mutations are implicated in CRC development [27], altered biochemical cellular metabolism of neoplastic cells, molecular heterogeneity between the primary CRC and its metastasis and the mysterious role of intra-tumoral inflammatory cellular infiltrate. All these multifactor could result in dissimilarities between the biological behavior of malignant cells regarding the manner of growth, invasion and metastasis. Moreover, this heterogeneity will reflect on the response of patients to therapy.
Therefore, the aim of this study is to investigate the roles of TSP50 as an oncoprotein with altered calcium metabolism and inflammatory cytokine namely SERCA2 and IL-8 respectively in CRA and CRC, understanding their roles in CRC initiation and propagation and the relation of these biomarkers’ expression to the intratumoral inflammatory infiltrate and other clinicopathological and prognostic factors.
As far as we know, this is the first study that correlates the immunohistochemical detection of TSP50, SERCA2 and IL-8 in CRA and CRC with clinicopathological factors.
Testes-specific protease 50 (TSP50) is a unique member of cancer/testis antigens, with exception of testes, it is not expressed in normal tissues. It is activated and re-expressed in human cancers such as breast cancer and other cancers [28].
In the present study the expression of TSP50 was examined in CRA, CRC as well as in normal colonic mucosa. It was found that TSP50 was not detected or weakly expressed in the normal mucosa tissues. Whereas TSP50 expression scores were significantly varied between CRA and CRC lesions. The significant increase of TSP50 expression found in CRC and CRA compared to normal mucosal tissues is of great value for considering TSP50 as a crucial protein for the process of tumorigenesis.
Also, the TSP50 expression in CRA neoplastic cells was tested in relation to clinicopathological parameters. It was found that high TSP50 expression was significantly identified in adenomas with tubulovillous architecture more than tubular adenomas and in adenomas with HGD more than those with LGD.
Regarding the TSP50 expression in CRC malignant cells and its relation to clinicopathological and prognostic factors, TSP50 overexpression in epithelial cells was observed more abundantly in moderately differentiated CRC than poorly differentiated tumors with no significant association between high TSP50 expression and tumor grade. On the other hand, TSP50 overexpression in CRC was significantly associated with advanced TNM staging and presence of LVI. Additionally, TSP50 protein upregulation was shown to be significantly associated with high intra-tumoral inflammatory infiltrate (both lymphocytes and neutrophils).
The findings of the present work supported the role of TSP50 in tumor cellular proliferation, migration and invasion reported by previous study which found that TSP50 mutation has led to diminished tumorigenic effect of TSP50 with subsequent decrease in in vitro cellular proliferation in nude mice [7]. Moreover, TSP50 epithelial overexpression was found to enhance the proliferation and invasion of cervical cancer cells via its protease activity [29]. Similarly, other study showed that TSP50 overexpression was significantly associated with poor prognostic outcomes in non-small cell lung cancer compared to those with low TSP50 expression [8]. Besides, high expression of TSP50 in neoplastic epithelial cells was associated with advanced malignancy and poor prognosis in human gastric cancer and colorectal carcinoma, respectively [24,30].
The interesting finding in the present work was the paradoxical low TSP50 epithelial expression in 54.5% of poorly differentiated CRC including the signet ring cell type and its high expression in associated non-neoplastic stromal cells; which may be attributed to the dual role that TSP50 plays in both epithelial neoplastic cells and stromal cells. Its epithelial expression enhances tumor cellular proliferation and migration, while the stromal expression of TSP50 facilitates the neoplastic epithelial cell invasion through its threonine protease activity. This could explain the heterogeneity of TSP50 epithelial and stromal expression in high grade CRC in the current work. Also, TSP50 expression was found to be variable depending on tumor type, genesis and microenvironment [31]. Along with the fact that malignant cells may alter or modify the expression of proteins that regulate cancer cell growth and invasion through epigenetic DNA hyper or hypomethylation or genetic mutations could add another point of explanation [32].
Intracellular Ca2+ is kept in a state of equilibrium and regulated by Ca2+ signaling and Ca2+ channels. Disruption of this equilibrium stimulates the expression of proteins that can lead to cellular transformation, proliferation, invasion and metastasis. One of the regulators of intracellular Ca2+ signaling is SERCA2 [13].
SERCA2 expression in CRA, CRC and normal mucosal tissues were investigated in the present study. It was observed that SERCA2 expression was variable between normal and neoplastic lesions (CRA and CRC) with significant increase in its expression in neoplastic lesions as none of the normal colonic tissues showed high SERCA2 expression. This was in agreement with previous study which found that the mean expression level of SERCA2 mRNA in malignant colorectal tissues was significantly higher than that in noncancerous tissues [25].
Furtherly, the SERCA2 expression in CRA neoplastic cells in relation to clinicopathological parameters was studied. It was found that high SERCA2 expression was significantly associated with adenoma with HGD than adenoma with LGD. No significant relation was detected between SERCA2 expression and histological architecture of adenoma.
Besides the fact that the expression of SERCA2 in CRC epithelial cells was tested, it was found that SERCA2 overexpression was significantly related to high tumor grade, advanced TNM staging, presence of LVI and high ITN (but not lymphocytic) infiltrate. These findings were similar to previous studies which showed that colorectal carcinoma with increased SERCA2 expression was significantly associated with serosal invasion, lymph node metastasis and advanced tumor stage compared to those with low SERCA2 expression meaning that SERCA2 may be a crucial biomarker in the development and progression of CRC [25, 33, 34].
In addition, previous studies reported the key role of intracellular calcium remodeling in colorectal cancer process that includes cellular proliferation, migration, invasion and metastasis [34, 35]. As during cellular transformation, disturbances of intracellular Ca2+ occur due to altered expression of Ca2+ channel proteins which control the process of Ca2+ pump [36]. One of these Ca2+ channel proteins is SERCA2 which is important for ER Ca2+ stores refill to maintain efficient protein folding and maturation, whereas overexpression causing ER Ca2+ stores overload that leads to ER stress observed in various cancers [37].
Our results supported the predominant association of SERCA2 overexpression with poor prognostic factors of CRC, as SERCA2 overexpression in cancer cells is a prerequisite for evasion of apoptosis causing depletion of intracellular Ca2+via increases Ca2+ influx into the ER with subsequent inactivation of caspases proved by previous study which showed that HCT-116 human colon cancer cells apoptosis can be triggered through increasing intracellular Ca2+ either by increasing extracellular Ca2+ influx or ER Ca2+ efflux [38], besides it enhances cellular proliferation, motility and invasion via the activation of mitogen-activated protein kinase (MAPK) signal transduction pathway through SERCA2 induced-ER stress which are essential for colorectal carcinogenesis [34, 39].
Furthermore, inhibition of SERCA2 expression was proved to induce G2/M cycle arrest and growth elimination in SW480 human colon adenocarcinoma epithelial cells in vitro and in vivo [40].
IL-8 is a multifunctional chemokine which was found to induce tumor growth, angiogenesis and metastatic potential in CRC.
IL-8 expression was examined in CRA, CRC as well as in normal colonic mucosa. A significant increase in IL-8 expression rate was detected in CRC more than in CRA and normal colonic mucosa. Similarly, previous studies found that IL-8 is upregulated in CRC compared to CRA and normal colonic mucosa [41, 42].
Subsequently, IL-8 expression was studied in CRA in relation to clinicopathological parameters, where no significant relations between IL-8 overexpression and CRA type or location were found, while high IL-8 immunostaining was significantly associated with CRA showing HGD. A Similar finding in other study which found that IL-8 mRNA level was significantly elevated with increased degree of dysplasia in the adenomas [42].
In addition, IL-8 expression in neoplastic cells of CRC was investigated, and it was detected that IL-8 protein overexpression was significantly linked to high tumor grade, presence of LVI, increased tumor size, regional lymph node, distant metastasis and high intra-tumoral inflammatory infiltrate. No significant relation was detected between IL-8 expression and tumor site or histological type.
Parallel to our results, previous studies showed that IL-8 expression is significantly upregulated in CRC neoplastic cells and this upregulation was associated with increased tumor growth, invasion and distant metastasis [43–46]. Added to that, human and mice in vitro colon cancer cells grew rapidly in IL-8 rich microenvironment with enhanced angiogenesis compared to normal microenvironment [26]. Moreover, IL-8 and its receptors promoted primary CRC progression and liver metastasis by direct effect on tumor cells, angiogenesis stimulation or regulating the tumor stroma, they also reported that high IL-8 levels in the tissues were significantly related to tumor grade, stage, lymph node and liver metastasis [47].
The growth signaling pathway (MAPK) is stimulated by interaction of IL-8 and its receptors that may be expressed in tumor associated stromal cells particularly the inflammatory cells [48], this explains the significant increase of IL-8 expression in relation to high intra-tumoral inflammatory infiltrate detected in the present study.
It is noteworthy that, in the current study, high expression of TSP50, SERCA2 and IL-8 were significantly associated with high intra-tumoral inflammatory infiltrate in CRC. This finding supports the idea of the role of inflammatory microenvironment in increasing mutation rates and enhancing the division of mutated cells. This may be due to release of reactive oxygen species (ROS) from activated inflammatory cells which are able to promote DNA mutation and genomic instability in the epithelial cells [49].
Regarding the correlation between the expression of TSP50, SERCA2 and IL-8 in CRC, significant positive correlation was found between the three biomarkers’ expression in neoplastic epithelial cells. This finding could be explained by the fact that both inflammatory process and tumorigenesis are related to disruption of Ca2+ homeostasis which is involved in DNA and gene methylation [36]. TSP50 gene abnormal methylation and the subsequent TSP50 protein expression in tumorous epithelial and stromal cells may be related to SERCA2 expression and intracellular Ca2+ alteration. Moreover, alteration of intracellular Ca2+ stimulates the secretion of pro-inflammatory cytokines from epithelial neoplastic cells as well as tumor associated stromal cells as a result of protein unfolding [50]. In addition to that, both SERCA2 and IL-8 have synergistic action through stimulating the MAPK growth signaling pathway in tumor cells [39, 48]. Thus the increased expression of IL-8 and SERCA2 in relation to high intratumoral inflammatory infiltrate with subsequent increase in TSP50 protein expression in CRC, in the present study, and the significant association of these biomarkers’ expression to adverse clinicopathological and prognostic factors namely: LVI and advanced TNM staging are of crucial value for understanding the role of these biomarkers in CRC initiation, growth and invasion.
Conclusion
Based on our findings. The aberrant expression of TSP50, SERCA2 and IL-8 in CRA and CRC compared to normal colonic mucosa could represent a different molecular transformation pathway. Also, increased expression of these biomarkers in neoplastic epithelial cells of CRC is associated with adverse prognostic factors through facilitating several hallmarks of cancer and could be considered as independent prognostic factors. Therefore, combination immunotherapy targeting TSP50, SERCA2 and IL-8 molecules would be a valuable effective therapy for inhibiting colorectal carcinoma initiation, proliferation and metastasis.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Tanta University Faculty of medicine. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
The authors confirm contribution to the paper as follows: study conception and design HY and MA; analysis and interpretation of results: MA; draft manuscript preparation: MA and DR. All authors reviewed the results and approved the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Ferlay J Ervik M Lam F Colombet M Mery L Piñeros M et al Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer (2020). Available from https://gco.iarc.fr/today/home (Accessed December 2020).
2.
Sung H Ferlay J Siegel RL Laversanne M Soerjmataram I Jemal A et al Erratum: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 70:313–41. 10.3322/caac.21609
3.
Jones RP Jackson R Dunne DFJ Malik HZ Fenwick SW Poston GJ et al Systematic Review and Meta-Analysis of Follow-Up after Hepatectomy for Colorectal Liver Metastases2. Br J Surg (2012) 99:477–86. 10.1002/bjs.8667
4.
Guinney J Dienstmann R Wang X de Reyniès A Schlicker A Soneson C et al The Consensus Molecular Subtypes of Colorectal Cancer. Nat Med (2015) 21:1350–6. 10.1038/nm.3967
5.
Shan J Yuan L Xiao Q Chiorazzi N Budman D Teichberg S et al TSP50, a Possible Protease in Human Testes, Is Activated in Breast Cancer Epithelial Cells. Cancer Res (2002) 62:290–4. 10.1371/journal.pone.0035030
6.
Cao Q-H Liu F Li C-Z Liu N Shu M Lin Y et al Testes-specific Protease 50 (TSP50) Promotes Invasion and Metastasis by Inducing EMT in Gastric Cancer. BMC Cancer (2018) 18:94–104. 10.1186/s12885-018-4000-y
7.
Song Z-B Bao Y-L Zhang Y Mi X-G Wu P Wu Y et al Testes-specific Protease 50 (TSP50) Promotes Cell Proliferation through the Activation of the Nuclear Factor κB (NF-Κb) Signalling Pathway. Biochem J (2011) 436:457–67. 10.1042/bj20101780
8.
Qiao W Shi B Han Y Tang HM Lin J Hu HY et al Testes-Specific Protease 50 and Non-Small Cell Lung Cancer Prognosis. Oncol Lett (2018) 15:8796–804. 10.3892/ol.2018.8387
9.
Ke J Lou J Zhong R Chen X Li J Liu C et al Identification of a Potential Regulatory Variant for Colorectal Cancer Risk Mapping to 3p21.31 in Chinese Population. Sci Rep (2016) 6:25194. 10.1038/srep25194
10.
Monteith GR McAndrew D Faddy HM Roberts-Thomson SJ . Calcium and Cancer: Targeting Ca2+ Transport. Nat Rev Cancer (2007) 7:519–30. 10.1038/nrc2171
11.
Roberts-Thomson SJ Chalmers SB Monteith GR . The Calcium-Signaling Toolkit in Cancer: Remodeling and Targeting. Cold Spring Harb Perspect Biol (2019) 11:a035204. 10.1101/cshperspect.a035204
12.
Arai M Otsu K MacLennan DH Periasamy M . Regulation of Sarcoplasmic Reticulum Gene Expression during Cardiac and Skeletal Muscle Development. Am J Physiology-Cell Physiol (1992) 262:C614–C620. 10.1152/ajpcell.1992.262.3.c614
13.
Papp S Dziak E Michalak M Opas M . Is All of the Endoplasmic Reticulum Created Equal? The Effects of the Heterogeneous Distribution of Endoplasmic Reticulum Ca2+-Handling Proteins. J Cel Biol (2003) 160:475–9. 10.1083/jcb.200207136
14.
Pierro C Sneyers F Bultynck G Roderick HL . ER Ca2+ Release and Store-Operated Ca2+ Entry - Partners in Crime or Independent Actors in Oncogenic Transformation?Cell Calcium (2019) 82:102061. 10.1016/j.ceca.2019.102061
15.
Wilson AJ Byron K Gibson PR . Interleukin-8 Stimulates the Migration of Human Colonic Epithelial Cells In Vitro. Clin Sci (1999) 97:385–90. 10.1042/cs0970385
16.
Sebok K Woodside D al-Aoukaty A Ho AD Gluck S Maghazachi AA . IL-8 Induces the Locomotion of Human IL-2-activated Natural Killer Cells. Involvement of a Guanine Nucleotide Binding (Go) Protein. J Immunol (1993) 150:1524–34.
17.
Masood R Cai J Tulpule A Zheng T Hamilton A Sharma S et al Interleukin 8 Is an Autocrine Growth Factor and a Surrogate Marker for Kaposi's Sarcoma. Clin Cancer Res (2001) 7:2693–702.
18.
Zhu YM Webster SJ Flower D Woll PJ . Interleukin-8/CXCL8 Is a Growth Factor for Human Lung Cancer Cells. Br J Cancer (2004) 91:1970–6. 10.1038/sj.bjc.6602227
19.
Rubie C Frick VO Pfeil S Wagner M Kollmar O Kopp B et al Correlation of IL-8 with Induction, Progression and Metastatic Potential of Colorectal Cancer. World J Gastroenterol (2007) 13(37):4996–5002. 10.3748/wjg.v13.i37.4996
20.
Vieth M Quirke P Lambert R Karsa LV Risio M . European Guidelines for Quality Assurance in Colorectal Cancer Screening and Diagnosis. First Edition–Annotations of Colorectal Lesions. Endoscopy (2012) 44(3):131–9. 10.1055/s-0032-1309798
21.
Hamilton SR Bosman FT Boffetta P Ilyas M Morreau H Nakamura SI et al Carcinoma of the colon and Rectum In: FTBosmanFCarneiroRHHrubanet al editors. WHO Classification of Tumors of the Digestive System. Lyon: IARC Press (2010). p. 134–46.
22.
American Joint Committee on Cancer. Colon and Rectum. In: MBAminSEdgeFGreeneet al editors. AJCC Cancer Staging Manual. 8th ed.New York, NY: Springer (2017).
23.
Wang F He W Jiang C Guo G Ke B Dai Q et al Prognostic Value of Inflammation-Based Scores in Patients Receiving Radical Resection for Colorectal Cancer. BMC Cancer (2018) 18:1102–11. 10.1186/s12885-018-4842-3
24.
Zheng L Xie G Duan G Yan X Li Q . High Expression of Testes-Specific Protease 50 Is Associated with Poor Prognosis in Colorectal Carcinoma. PLoS ONE (2011) 6(7):e22203. 10.1371/journal.pone.0022203
25.
Chung F-Y Lin S-R Lu C-Y Yeh C-S Chen F-M Hsieh J-S et al Sarco/Endoplasmic Reticulum Calcium-ATPase 2 Expression as a Tumor Marker in Colorectal Cancer. Am J Surg Pathol (2006) 30(8):969–74. 10.1097/00000478-200608000-00006
26.
Lee YS Choi I Ning Y Kim NY Khatchadourian V Yang D et al Interleukin-8 and its Receptor CXCR2 in the Tumour Microenvironment Promote colon Cancer Growth, Progression and Metastasis. Br J Cancer (2012) 106:1833–41. 10.1038/bjc.2012.177
27.
Bhalla A Zulfiqar M Bluth MH . Molecular Diagnostics in Colorectal Carcinoma. Clin Lab Med (2018) 38:311–42. 10.1016/j.cll.2018.02.008
28.
Wang Y Yang M Bao Y Li Y . The TSP50 Gene Is Re-expressed in Different Types of Human Cancers. In: Proceedings 2011 International Conference on Human Health and Biomedical Engineering; August 2011; Jilin, China (2011). p. 1300–2.
29.
Yuan J Wu C Huang M Zhou J Ben W Zhang G . TSP50 Depends on its Threonine Protease Activity and its Interactions with TNF-α-Induced NF-Κb for its Role in Human Cervical Tumorigenesis. Cell Biochem Biophys (2015) 71(2):891–6. 10.1007/s12013-014-0279-8
30.
Liu F Cao Q Liu N Li C You C Liu C et al Overexpression of Testes-specific Protease 50 (TSP50) Predicts Poor Prognosis in Patients with Gastric Cancer. Gastroenterol Res Pract (2014) 2014:1–7. 10.1155/2014/498246
31.
Xu H-P Yuan L Shan J Feng H . Localization and Expression of TSP50 Protein in Human and Rodent Testes. Urology (2004) 64:826–32. 10.1016/j.urology.2004.05.012
32.
Lee C-J Ahn H Jeong D Pak M Moon JH Kim S . Impact of Mutations in DNA Methylation Modification Genes on Genome-wide Methylation Landscapes and Downstream Gene Activations in Pan-Cancer. BMC Med Genomics (2020) 13:27–40. 10.1186/s12920-020-0659-4
33.
Evangelista AM Thompson MD Weisbrod RM Pimental DR Tong X Bolotina VM et al Redox Regulation of SERCA2 Is Required for Vascular Endothelial Growth Factor-Induced Signaling and Endothelial Cell Migration. Antioxid Redox Signaling (2012) 17:1099–108. 10.1089/ars.2011.4022
34.
Fan L Li A Li W Cai P Yang B Zhang M et al Novel Role of Sarco/endoplasmic Reticulum Calcium ATPase 2 in Development of Colorectal Cancer and its Regulation by F36, a Curcumin Analog. Biomed Pharmacother (2014) 68(8):1141–8. 10.1016/j.biopha.2014.10.014
35.
Dang D Rao R . Calcium-ATPases: Gene Disorders and Dysregulation in Cancer. Biochim Biophys Acta (2016) 1863(6 Pt B):1344–50. 10.1016/j.bbamcr.2015.11.016
36.
Izquierdo-Torres E Hernández-Oliveras A Fuentes-García G Zarain-Herzberg Á . Calcium Signaling and Epigenetics: A Key point to Understand Carcinogenesis. Cell Calcium (2020) 91:102285. 10.1016/j.ceca.2020.102285
37.
Zhong T Pan X Wang J Yang B Ding L . The Regulatory Roles of Calcium Channels in Tumors. Biochem Pharmacol (2019) 169:113603. 10.1016/j.bcp.2019.08.005
38.
Lu M Sun L Zhou J Zhao Y Deng X . Dihydroartemisinin-induced Apoptosis Is Associated with Inhibition of Sarco/endoplasmic Reticulum Calcium ATPase Activity in Colorectal Cancer. Cel Biochem Biophys (2015) 73:137–45. 10.1007/s12013-015-0643-3
39.
Krebs J Agellon LB Michalak M . Ca2+ Homeostasis and Endoplasmic Reticulum (ER) Stress: An Integrated View of Calcium Signaling. Biochem Biophysical Res Commun (2015) 460:114–21. 10.1016/j.bbrc.2015.02.004
40.
Yang B Zhang M Gao J Li J Fan L Xiang G et al Small Molecule RL71 Targets SERCA2 at a Novel Site in the Treatment of Human Colorectal Cancer. Oncotarget (2015) 6:37613–25. 10.18632/oncotarget.6068
41.
Asfaha S Dubeykovskiy AN Tomita H Yang X Stokes S Shibata W et al Mice that Express Human Interleukin-8 Have Increased Mobilization of Immature Myeloid Cells, Which Exacerbates Inflammation and Accelerates colon Carcinogenesis. Gastroenterology (2013) 144:155–66. 10.1053/j.gastro.2012.09.057
42.
Cui G Yuan A Goll R Vonen B Florholmen J . Dynamic Changes of Interleukin-8 Network along the Colorectal Adenoma-Carcinoma Sequence. Cancer Immunol Immunother (2009) 58:1897–905. 10.1007/s00262-009-0702-y
43.
Xia W Chen W Zhang Z Wu D Wu P Chen Z et al Prognostic Value, Clinicopathologic Features and Diagnostic Accuracy of Interleukin-8 in Colorectal Cancer: a Meta-Analysis. PloS one (2015) 10(4):e0123484. 10.1371/journal.pone.0123484
44.
Wei H Li J Xie M Lei R Hu B . Comprehensive Analysis of Metastasis-Related Genes Reveals a Gene Signature Predicting the Survival of colon Cancer Patients. Peer J (2018) 6:e5433. 10.7717/peerj.5433
45.
Fisher RC Bellamkonda K Alex Molina L Xiang S Liska D Sarvestani SK et al Disrupting Inflammation-Associated CXCL8-CXCR1 Signaling Inhibits Tumorigenicity Initiated by Sporadic- and Colitis-Colon Cancer Stem Cells. Neoplasia (2019) 21:269–81. 10.1016/j.neo.2018.12.007
46.
Shimizu M Tanaka N . IL-8-induced O-GlcNAc Modification via GLUT3 and GFAT Regulates Cancer Stem Cell-like Properties in colon and Lung Cancer Cells. Oncogene (2019) 38:1520–33. 10.1038/s41388-018-0533-4
47.
Bie Y Ge W Yang Z Cheng X Zhao Z Li S et al The Crucial Role of CXCL8 and its Receptors in Colorectal Liver Metastasis. Dis Markers (2019) 2019:1–12. 10.1155/2019/8023460
48.
Chan LP Liu C Chiang FY Wang LF Lee KW Chen WT et al IL-8 Promotes Inflammatory Mediators and Stimulates Activation of P38 MAPK/ERK-NF-κB Pathway and Reduction of JNK in HNSCC. Oncotarget (2017) 8(34):56375–88. 10.18632/oncotarget.16914
49.
Hussain SP Hofseth LJ Harris CC . Radical Causes of Cancer. Nat Rev Cancer (2003) 3:276–85. 10.1038/nrc1046
50.
Luo B Lee AS . The Critical Roles of Endoplasmic Reticulum Chaperones and Unfolded Protein Response in Tumorigenesis and Anticancer Therapies. Oncogene (2013) 32:805–18. 10.1038/onc.2012.130
Summary
Keywords
clinicopathological factors, colorectal adenoma, colorectal carcinoma, immunohistochemistry, IL-8, SERCA2 TSP50
Citation
Youssef HMK, Radi DA and Abd El-Azeem MA (2021) Expression of TSP50, SERCA2 and IL-8 in Colorectal Adenoma and Carcinoma: Correlation to Clinicopathological Factors. Pathol. Oncol. Res. 27:1609990. doi: 10.3389/pore.2021.1609990
Received
30 July 2021
Accepted
05 October 2021
Published
21 October 2021
Volume
27 - 2021
Edited by
Andrea Ladányi, National Institute of Oncology (NIO), Hungary
Updates
Copyright
© 2021 Youssef, Radi and Abd El-Azeem.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marwa A. Abd El-Azeem, marwaabdelhaq@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.