Abstract
Background:
Angiogenesis is closely associated with tumor growth and metastasis, and microvascular density (MVD) is currently the clinical standard for evaluating tumor angiogenesis. Thus, the detection of intratumoral MVD is of great significance for understanding disease progression and predicting patient prognosis.
Methods:
Tumor tissue sections of 238 patients with lung adenocarcinoma (LUAD) who underwent radical surgery were retrospectively analyzed. Immunohistochemical (IHC) staining was carried out using a CD34 polyclonal antibody to determine intratumoral MVD, and the relationship of CD34-MVD with the clinicopathological characteristics and survival time of LUAD patients was analyzed.
Results:
CD34-MVD was associated with tumor size, lymph node metastasis, tumor recurrence, and patient survival status; patients with tumor size ≤3 cm (P = 0.015), negative for lymph node metastasis (P = 0.049), no tumor recurrence (P = 0.021), and survival (P = 0.042) had higher MVD. Survival analysis suggested that patients with high MVD had higher disease-free survival (log-rank P = 0.005) and overall survival (log-rank P = 0.004) compared to patients with low MVD. The Cox proportional hazards model showed that a high MVD (P = 0.022) reduced the risk of postoperative tumor recurrence in patients with LUAD.
Conclusion:
Decreased intratumoral CD34 positive microvessels were associated with tumor development in patients with LUAD. CD34-MVD is an independent risk factor affecting postoperative tumor recurrence in patients with LUAD and can be used as a prognostic indicator for this group of patients.
Introduction
Lung cancer is currently the most common malignancy with the highest mortality rate worldwide [1]. In China, lung cancer ranks first among all malignancies in terms of incidence and mortality rate [2]. Lung adenocarcinoma (LUAD) is the most common pathological type of lung cancer, accounting for approximately 45% of all cases, and has a significantly higher incidence than other pathological subtypes such as squamous cell, small cell, and large cell carcinomas [3]. Surgical resection is the mainstay of treatment for early to intermediate stage (stage I–IIIA) nonsmall cell lung cancer (NSCLC), but the risk of tumor recurrence and death after radical surgery remains high [4]. Therefore, the search for molecular markers associated with tumor progression or prognosis in lung cancer is of great significance for elucidating tumor pathogenesis and screening patients with poor prognosis [5, 6].
Tumor growth is accompanied by angiogenesis, and antiangiogenic therapy has always been a crucial target in antitumor treatment [7]. The relationship between tumor angiogenesis and the prognosis of tumor patients has also been widely studied and is currently a hot topic of discussion. Microvascular density (MVD) is an indicator used to assess the number of blood vessels within a tumor [8] and plays an important role in predicting the prognosis of patients with tumors [9–11].
CD34 is a pan-endothelial cell marker commonly used to assess tumor vascularity. Although researchers have used CD34 antibodies to label the tumor microvasculature by performing immunohistochemical (IHC) staining of lung cancer tumor tissues and to assess the relationship between MVD, tumor progression, and patient prognosis, the results of different studies have been controversial. A study by Tanaka et al. [12] that included 236 NSCLC patients found that intratumoral CD34-MVD was not associated with tumor stage or five-year survival rate. Similarly, Donnem et al. found that among 335 NSCLC patients, high vs. low CD34-MVD was not associated with a five-year survival rate [13]. In contrast, Kadota et al. found that among 147 NSCLC patients, those with high CD34-MVD had lower survival rates [14]. In a study of 81 patients with NSCLC, Bing et al. showed that an increase in CD34-MVD was associated with tumor progression but not lymph node metastasis; unfortunately, the study did not include a survival analysis [15]. The results of the study by Pomme et al., which included 371 NSCLC patients, were contrary to all the studies above and instead showed that the increase in CD34-MVD was associated with lower TNM stage and better prognosis [16]. Furthermore, all the above studies included NSCLC patients, and none analyzed patients with lung adenocarcinoma separately. Therefore, the relationship between CD34-MVD and tumor progression and prognosis in patients with lung adenocarcinoma remains inconclusive and warrants further investigation.
In this study, IHC staining was performed using CD34 antibodies on tumor tissue sections from 238 LUAD patients who underwent radical surgery to determine intratumoral MVD and to analyze the relationship between CD34-MVD and patient clinicopathological characteristics and survival. Based on the above, we aimed to explore the role of CD34-MVD in tumor development and its prognostic value.
Materials and methods
Patients
Patients enrolled in this study were required to meet the following conditions: 1) a definitive pathological diagnosis of LUAD with no history of other tumors; 2) no neoadjuvant treatments, such as chemotherapy and radiotherapy, before surgery; 3) radical surgery for lung cancer to ensure complete tumor resection; 4) postoperative stage within stages I–III, excluding patients with distant metastases; and 5) postoperative tumor tissues formally sampled by the Department of Pathology and used to prepare tissue wax blocks, which were well preserved and usable.
In strict accordance with the criteria above, we screened and enrolled 238 patients who underwent radical lung cancer surgery between 1 January 2011, and 30 December 2015, at the Second Affiliated Hospital of Zhejiang University School of Medicine. The age of these patients ranged from 26 to 87 years, with a mean age of 60.48 years. The distributions of other characteristics are detailed in Table 1.
TABLE 1
| Grouping | Number (proportion %) | MVD( ± s) | Difference value | 95% CI | T test/F test | |
|---|---|---|---|---|---|---|
| T/F value | P value | |||||
| Age, years | ||||||
| ≤60 | 108 (45.4%) | 20.16 ± 6.414 | −0.235 | −1.867∼1.398 | −0.283 | 0.777 |
| >60 | 130 (54.6%) | 20.39 ± 6.323 | ||||
| Sex | ||||||
| Female | 133 (55.9%) | 20.77 ± 6.450 | 1.091 | −0.540∼2.722 | 1.317 | 0.189 |
| Male | 105 (44.1%) | 19.68 ± 6.203 | ||||
| Smoking | ||||||
| Never | 166 (69.7%) | 20.41 ± 6.531 | 0.410 | −1.359∼2.178 | 0.456 | 0.649 |
| Have | 72 (30.3%) | 20.00 ± 5.953 | ||||
| Tumor Site | ||||||
| Left lung | 106 (44.5%) | 19.78 ± 6.123 | −0.906 | −2.538∼0.725 | −1.095 | 0.275 |
| Right lung | 132 (55.5%) | 20.68 ± 6.526 | ||||
| Tumor Differentiation | ||||||
| Well | 59 (24.8%) | 21.12 ± 5.986 | — | 19.56∼22.68 | 2.055 | 0.130 |
| Moderately | 85 (35.7%) | 20.84 ± 6.766 | 19.38∼22.29 | |||
| Poorly | 94 (39.5%) | 19.27 ± 6.112 | 18.01∼20.52 | |||
| Pathological Subtypes | ||||||
| Acinar | 127 (53.4%) | 20.69 ± 6.699 | — | 19.52∼21.87 | 2.013 | 0.078 |
| Lepidic | 35 (14.7%) | 21.69 ± 5.718 | 19.72∼23.65 | |||
| Micropapillary | 9 (3.8%) | 21.78 ± 4.919 | 18.00∼25.56 | |||
| Papillary | 31 (13.0%) | 18.55 ± 5.525 | 16.52∼20.57 | |||
| Solid | 18 (7.6%) | 20.22 ± 6.967 | 16.76∼23.69 | |||
| Variant | 18 (7.6%) | 17.00 ± 5.224 | 14.40∼19.60 | |||
| Tumor size | ||||||
| ≤3 cm | 131 (55.0%) | 21.34 ± 5.887 | 2.000 | 0.393∼3.608 | 2.451 | 0.015 |
| >3 cm | 107 (45.0%) | 19.34 ± 6.626 | ||||
| T Stage | ||||||
| T1-2 | 186 (78.2%) | 20.34 ± 6.224 | 0.243 | −1.724∼2.209 | 0.243 | 0.808 |
| T3-4 | 52 (21.8%) | 20.10 ± 6.849 | ||||
| N Stage | ||||||
| N0 | 132 (55.5%) | 21.00 ± 6.699 | 1.604 | 0.006∼3.201 | 1.978 | 0.049 |
| N+ | 106 (44.5%) | 19.40 ± 5.801 | ||||
| TNM Stage | ||||||
| Stage I | 74 (31.1%) | 21.54 ± 6.513 | — | 20.03∼23.05 | 2.860 | 0.059 |
| Stage II | 73 (30.7%) | 19.05 ± 6.506 | 17.54∼20.57 | |||
| Stage III | 91 (38.2%) | 20.25 ± 5.955 | 19.01∼21.49 | |||
| Tumor Recurrence | ||||||
| Yes | 122 (51.3%) | 19.36 ± 5.972 | −1.898 | −2.325∼−0.290 | −2.325 | 0.021 |
| No | 116 (48.7%) | 21.26 ± 6.615 | ||||
| Status of Survival | ||||||
| Death | 63 (26.5%) | 18.89 ± 5.949 | −1.900 | −3.726∼−0.073 | −2.049 | 0.042 |
| Survival | 175 (73.5%) | 20.79 ± 6.433 | ||||
Relationship between MVD and clinicopathologic characteristics.
CI, confidence interval; MVD, microvascular density. Significant P values are presented in bold.
Information acquisition and follow-up
The clinicopathological characteristics of all patients were collected using the hospital information system. These included patient name, sex, age, smoking status, tumor location, tumor size, degree of differentiation, pathological subtype, and TNM stage. Postoperative TNM staging of all patients with LUAD was performed according to the 8th edition of the Union for International Cancer Control staging criteria.
Follow-up was conducted through a combination of accessing the hospital information system (outpatient and inpatient medical records) and telephone consultation. Follow-up information included whether the patients experienced tumor recurrence, whether they survived, and the time to a positive event after radical lung cancer surgery. Disease-free survival (DFS) was calculated as the time from complete resection to tumor recurrence. Overall survival (OS) was calculated as the time from the patient’s diagnosis of LUAD to death.
Research ethics
This study was approved by the Ethics Committee of the Second Affiliated Hospital of the Zhejiang University School of Medicine (Document batch number 2019-308). Informed consent was waived by our Ethics Committee because of the retrospective nature of our study. We confirm that all methods were performed in accordance with the relevant guidelines and regulations.
IHC staining
Tumor tissue wax blocks were sliced to obtain tissue sections with a thickness of 3–5 μm.
The tissue slides were deparaffinized and hydrated using xylene, ethanol, and distilled water in turn. Antigen retrieval: Citrate buffer (PH 6.0) was poured into the pressure cooker and heated; After boiling, the slides were added and heated for 2 min under closed conditions; The slides were washed with phosphate-buffered saline (PBS) (PH7.4) after cooling. Quenching endogenous peroxidase activity: Incubate slides with 3% H2O2 solution for 10 min at room temperature; After washing with PBS (PH7.4), serum blocking was performed by adding 3% bovine serum albumin (Haokebio, HK5021, Hangzhou, China) for 30 min at room temperature. Antibody incubation: Primary antibody CD34 (Proteintech, 14486-1-ap, Wuhan, China) prepared with PBS (PH7.4) at a concentration ratio of 1:800 was added and incubated for 90 min. After washing with PBS (PH7.4), ultrasensitive rabbit and mouse universal secondary antibodies (Biolynx, I20012B, Hangzhou, China) was added and incubated for 30 min. Chromogenic reaction: After washing with PBS (PH7.4), diaminobenzidine chromogenic solution (Biolynx, I20012C, Hangzhou, China) was added (diluent and concentrate were prepared at a ratio of 1,000:50) and incubated for 2–5 min. Positive color reactions appeared brownish-yellow, and the color development was terminated by rinsing with pure water. After color development, hematoxylin staining solution was used to stain the nuclei. Finally, the slides were dehydrated and mounted. After microscopic examination, the section images were scanned and captured using a digital pathology slide scanner (KFBIO, KF-PRO-120, Ningbo, China).
Microvasculature interpretation and MVD counting
The sections were read using digital slice-reading software K-VIEWER (KFBIO, ver1.7.1.1, Ningbo, China). Based on the Weidner correction method [17], any single endothelial cell or cell mass stained by the antibody, whether it formed a lumen, was considered to be a countable microvessel as long as it was clearly demarcated from the surrounding microvasculature, tumor cells, and other connecting tissues. Microvessels within sclerotic areas of the tumor and soft tissues at the tumor margins were not counted. Vessels with smooth muscle walls and lumen diameters greater than eight red blood cells were also excluded. For each specimen, three areas with the highest number of microvessels were selected at low power (×10), that is, “hotspots.” The number of microvessels was counted for each high-power field (HPF, ×40), and the average value was taken as the MVD value. All procedures were performed independently by two experienced pathologists.
Statistical analysis
Data were analyzed using the statistical package SPSS 25.0 and R version 4.2.1. The t-test or F-test was used to analyze the association between MVD and the clinicopathological characteristics. The Kaplan-Meier method was used to calculate the survival rate, and the log-rank test was used to compare differences in survival. Cox proportional hazards regression analysis was used for univariate and multivariate analyses to predict survival. Differences were considered statistically significant at P < 0.05.
Results
CD34 positive microvessels in LUAD
CD34 positive microvessels in LUAD is shown in Figure 1. MVD ranged between 8 and 38 vessels per HPF (×40), with a mean of 20.29 ± 6.352 vessels. Using the mean value as a cutoff, patients with an MVD ≤20 were assigned to the low-MCD group and those with an MVD >20 to the high-MVD group.
FIGURE 1
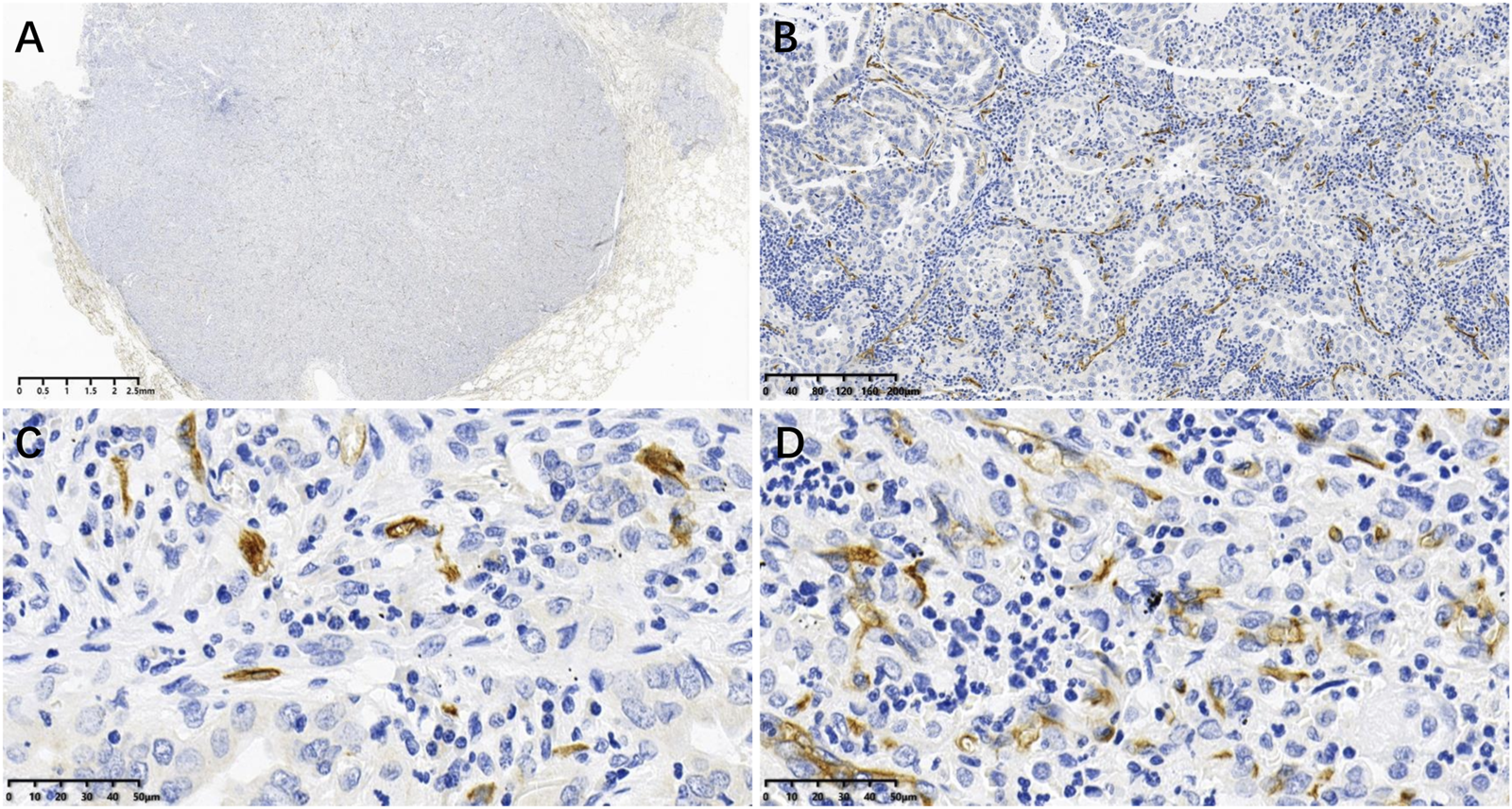
CD34 positive microvessels in LUAD tissues: (A) Image acquired by digital pathology scanner; (B) CD34 positive microvessels at low power (×10); (C, D) CD34 positive microvessels at high power (×40), where (C) is low MVD (MVD ≤20 vessels) and D is high MVD (MVD >20 vessels).
Relationship between MVD and clinicopathologic features
When grouped by tumor size, patients in the ≤3 cm group had an MVD of 21.34 ± 5.887, while those in the >3 cm group had an MVD of 19.34 ± 6.626, with the ≤3 cm group showing a higher MVD (T = 2.451, P = 0.015). When grouped by N-stage, patients without lymph node metastasis (N0) had an MVD of 21.00 ± 6.699, while those with lymph node metastasis (N+) had an MVD of 19.40 ± 5.801, with the N0 group showing a higher MVD (T = 1.978, P = 0.049). When grouped by tumor recurrence, patients in the recurrence group had an MVD of 19.36 ± 5.972, while those in the non-recurrence group had an MVD of 21.26 ± 6.615, and the recurrence group showed a lower MVD (T = −2.325, P = 0.021). When grouped by mortality status, patients in the deceased group had an MVD of 18.89 ± 5.949, while those in the non-deceased group had an MVD of 20.79 ± 6.433, with the deceased group showing a lower MVD (T = −2.049, P = 0.042).
There was no statistically significant difference in MVD when the patients were grouped by age, sex, smoking status, tumor location, degree of differentiation, T stage, and TNM stage (P ≥ 0.05).
CD34 positive microvessels in different pathological subtypes
LUAD can be divided according to its growth pattern into five main pathological subtypes: lepidic, papillary, micropapillary, acinar, and solid. Based on the data in Table 1, we found that the MVD was much lower in the papillary subtype and variant subtype. Representative images of CD34 positive microvessels in each pathological subtype are shown in Figure 2.
FIGURE 2
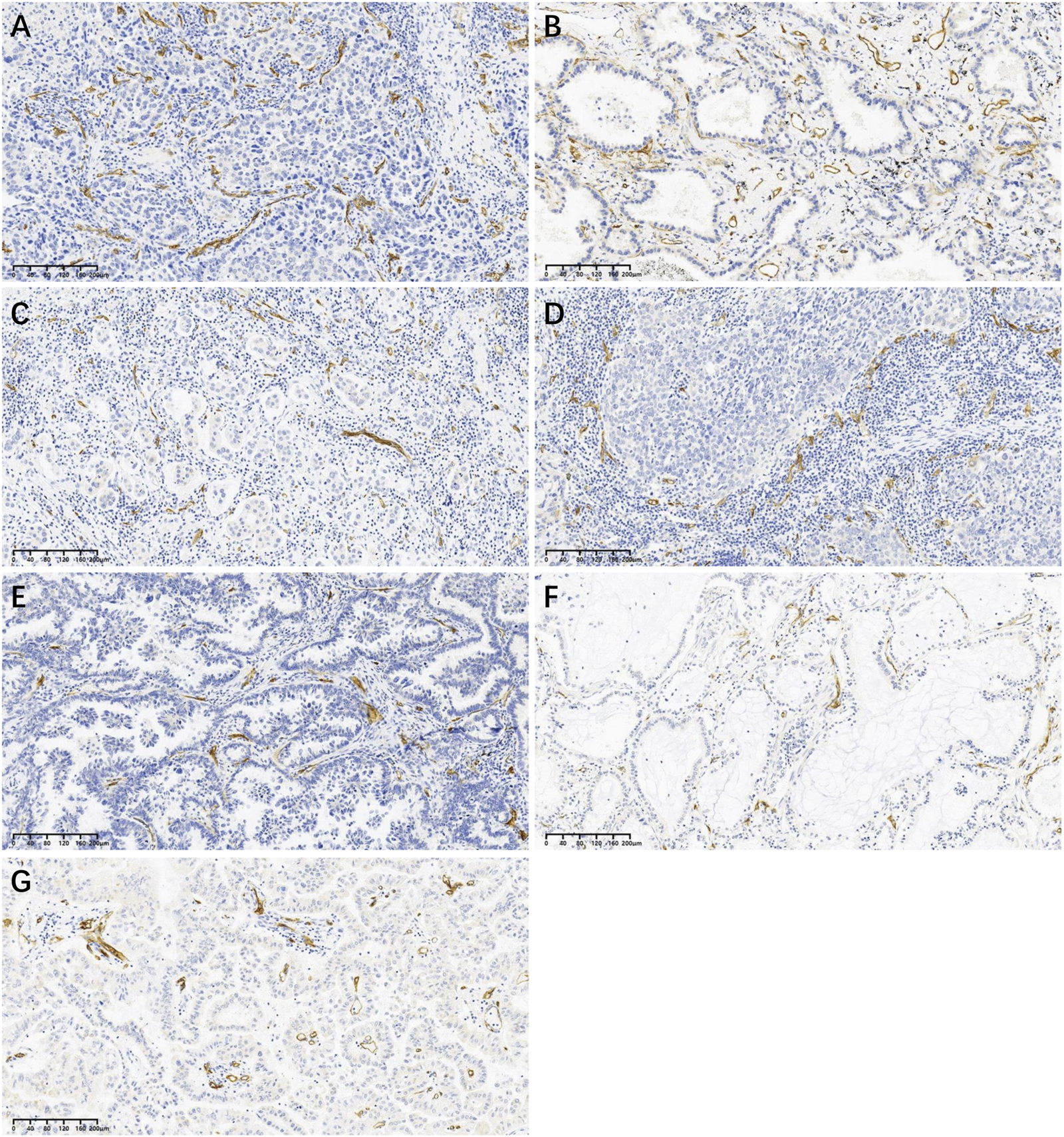
Representative images of CD34 positive microvessels in each pathological subtype at low power (×10): (A) Acinar subtype; (B) Lepidic subtype; (C) Micropapillary subtype; (D) Solid subtype; (E) Papillary subtype; (F) Mucinous adenocarcinoma (one of variant subtypes); (G) Enteric adenocarcinoma (one of variant subtypes).
Survival analysis
The five-year DFS rates of patients in the low and high MVD groups were 38.0% and 54.2%, respectively, with the high MVD group showing a higher DFS rate than the low MVD group (log-rank P = 0.005) (Figure 3A). The five-year OS rates of patients in the low and high MVD groups were 63.4% and 81.2%, respectively, with the high MVD group showing a higher OS rate than the low MVD group (log-rank P = 0.004) (Figure 3B).
FIGURE 3
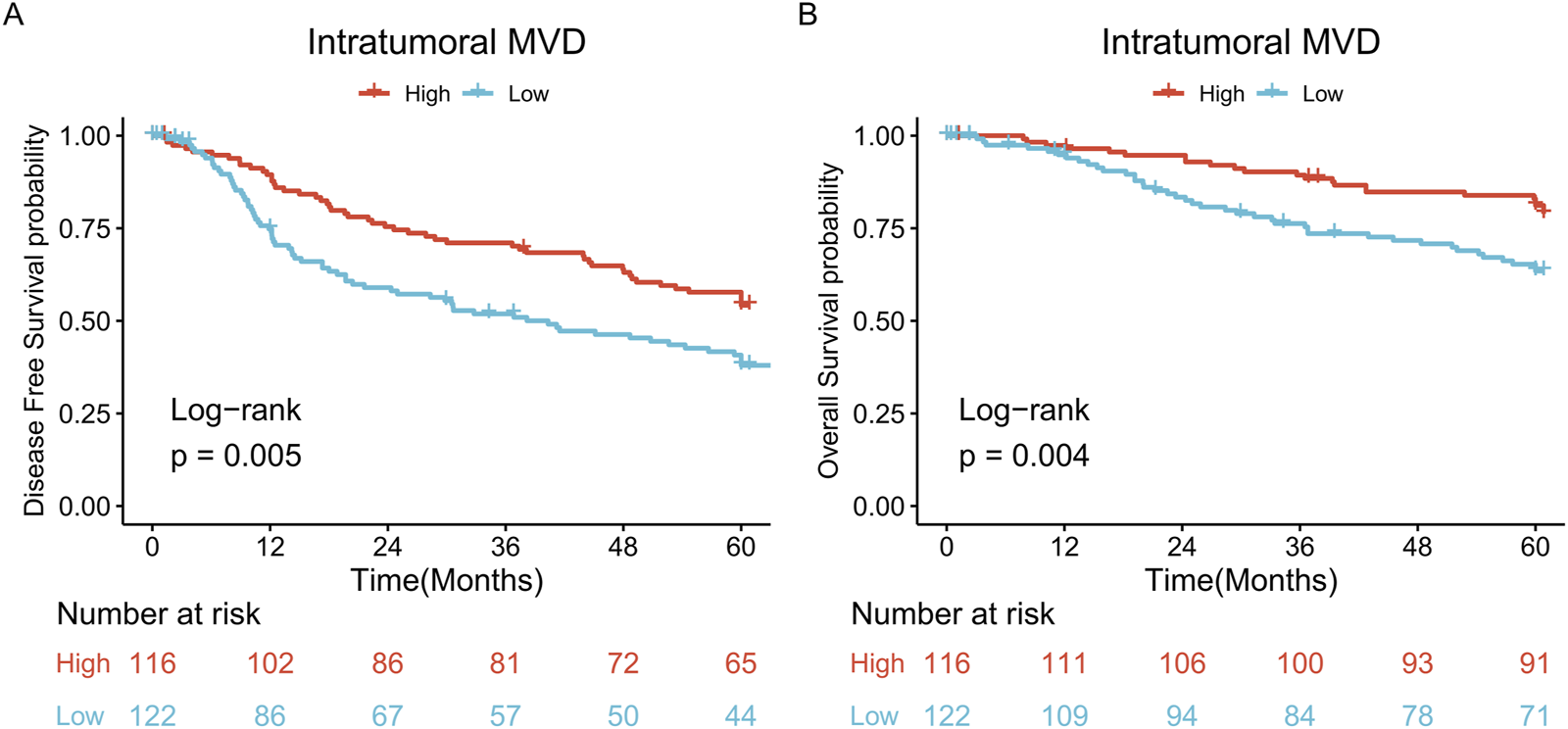
Survival analysis. (A) DFS curves of patients in the high and low MVD groups (log-rank P = 0.005); (B) OS curves of patients in the high and low MVD groups (log-rank P = 0.004).
Cox proportional hazards regression analysis
Cox proportional hazards regression was used to perform univariate survival analysis (including DFS and OS) for each grouping variable. The results indicated that age, sex, smoking status, tumor location, tumor size, and T stage were not associated with DFS or OS (P > 0.05). In contrast, lymph node metastasis status (P < 0.001), TNM stage (P < 0.001), degree of differentiation (P < 0.001), tumor recurrence (P < 0.001), and MVD (P = 0.005) were associated with patient survival.
Next, the positive variables were included in the construction of a multivariate Cox proportional hazards model for tumor recurrence and death after radical surgery in patients with LUAD. The results showed that the degree of differentiation (HR = 1.867, 95% CI = 1.444–2.414, P < 0.001), lymph node metastasis (HR = 1.779, 95% CI = 1.090–2.904, P = 0.021), and MVD (HR = 0.655, 95% CI = 0.456–0.941, P = 0.022) had statistically significant effects on DFS. This implies that poorer differentiation and the presence of lymph node metastasis increase the risk of tumor recurrence after radical surgery in patients with LUAD. In contrast, high MVD decreases the risk of tumor recurrence. Details are presented in Table 2.
TABLE 2
| Variables | DFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | |
| Age, year | ||||||||
| ≤60* | ||||||||
| >60 | 0.599 | 1.100 (0.771–1.571) | — | — | 0.204 | 1.387 (0.837–2.297) | — | — |
| Sex | ||||||||
| Female* | ||||||||
| Male | 0.906 | 1.022 (0.714–1.463) | — | — | 0.109 | 1.498 (0.914–2.456) | — | — |
| Smoking | ||||||||
| Never* | ||||||||
| Have | 0.488 | 0.868 (0.582–1.294) | — | — | 0.520 | 1.190 (0.700–2.024) | — | — |
| Tumor Site | ||||||||
| Left lung* | ||||||||
| Right lung | 0.164 | 1.293 (0.900–1.858) | — | — | 0.906 | 0.971 (0.590–1.596) | — | — |
| Tumor Size | ||||||||
| ≤3 cm* | ||||||||
| >3 cm | 0.331 | 0.837 (0.584–1.198) | — | — | 0.847 | 0.952 (0.579–1.566) | — | — |
| T Stage | ||||||||
| T1-2* | ||||||||
| T3-4 | 0.722 | 0.924 (0.599–1.426) | — | — | 0.751 | 1.098 (0.615–1.962) | — | — |
| N Stage | ||||||||
| N0* | ||||||||
| N1-3 | <0.001 | 2.949 (2.045–4.251) | 0.021 | 1.779 (1.090–2.904) | <0.001 | 3.683 (2.148–6.317) | 0.808 | 1.099 (0.513–2.354) |
| TNM Stage | <0.001 | 1.704 (1.358–2.138) | 0.172 | 1.242 (0.910–1.695) | <0.001 | 1.896 (1.360–2.642) | 0.446 | 1.203 (0.748–1.937) |
| Stage I | ||||||||
| Stage II | ||||||||
| Stage III | ||||||||
| Tumor Differentiation | <0.001 | 2.142 (1.668–2.750) | <0.001 | 1.867 (1.444–2.414) | <0.001 | 4.041 (2.536–6.438) | <0.001 | 3.053 (1.803–5.171) |
| Well | ||||||||
| Moderately | ||||||||
| Poorly | ||||||||
| Tumor Recurrence | ||||||||
| No* | ||||||||
| Yes | — | — | — | — | <0.001 | 24.840 (7.780–79.310) | <0.001 | 14.275 (4.389–46.432) |
| MVD Grouping | ||||||||
| Low* | ||||||||
| High | 0.005 | 0.600 (0.419–0.859) | 0.022 | 0.655 (0.456–0.941) | 0.005 | 0.474 (0.282–0.796) | 0.188 | 0.702 (0.415–1.189) |
Univariate and multivariate analysis of DFS and OS.
CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; MVD, microvascular density; OS, Overall survival.
Note: * As control group; Significant P values are presented in bold.
Discussion
Intratumoral blood vessels provide oxygen and nutrients to tumor cells; hence, angiogenesis is thought to play an important role in tumor development. However, the processes and mechanisms underlying tumor neovascularization are complex. They may include sprouting angiogenesis, intussusceptive angiogenesis, vasculogenesis, recruitment of endothelial progenitor cells, vascular mimicry, and transdifferentiation of cancer stem cells [18]. Studies identifying various aspects of tumor angiogenesis, especially biomarkers targeting tumor angiogenesis, can help us better understand its molecular mechanisms and discover targets for designing effective anti-tumor therapies [19]. The selection of appropriate tumor vascular markers for MVD counting enables quantitative morphological analysis of the tumor vasculature and reflects the extent of tumor angiogenesis [8]. CD34, as a powerful pan-endothelial cell marker, is widely expressed in mature vascular endothelial cells and has garnered extensive interest among clinicians in clinical practice, especially in the fields of cardiovascular and cerebrovascular diseases and cancer [20]. This was the starting point of our study, in which the abundance of microvessels within LUAD tumors was quantified using CD34-MVD, and the relationship between CD34-MVD, clinicopathological characteristics, and patient prognosis was analyzed.
Our findings revealed that CD34-MVD was higher in patients with tumor size ≤3 cm than in those with tumor size >3 cm, and higher in patients without lymph node metastasis than in those with lymph node metastasis. This result reflects the fact that in LUAD, CD34 positive microvessels are more abundant in the early tumor microenvironment, which then decreases in abundance as the tumor progresses (tumor enlargement and lymph node metastasis). Our results are similar to those of Carlini et al. [21] who found that intratumoral CD34-MVD was lower in patients with stage T2 NSCLC and higher in those with stage T1 NSCLC. We analyzed two possible reasons for this result. First, CD34 positive microvessels in lung cancer tumor tissues as a differentiated microvessels tpye, which was more likely to display intratumoral mature blood vessels and peritumoral normal blood vessels [22]. Second, CD34-MVD was also shown to be associated with intratumoral necrosis, resulting in uneven oxygen distribution [16]. Thus, we hypothesized that, as the tumor increased in size, there was a decrease in mature differentiated blood vessels, an increase in intratumoral necrosis, and the occurrence of tumor hypoxia, which in turn promoted the metastasis of lung cancer to the lymph nodes. Therefore, a reduction in CD34 positive microvessels may be associated with tumor development in LUAD.
According to the survival analysis conducted in this study, patients with high CD34-MVD showed better prognosis for both DFS and OS. High CD34-MVD reduced the risk of tumor recurrence in a multivariate Cox proportional hazards regression model of postoperative tumor recurrence. Since we previously found that the decrease in CD34-MVD was associated with tumor invasion events (tumor enlargement and lymph node metastasis), thus explaining the favorable prognosis of patients with high CD34-MVD and the poor prognosis of those with low CD34-MVD. This detrimental effect of reduced CD34-MVD on prognosis has been found not only in NSCLC [16] but also in other malignancies, such as kidney cancer [23], bladder cancer [24], ovarian cancer [25], intrahepatic cholangiocarcinoma [26], and hepatocellular carcinoma [27]. Therefore, we suggest that CD34-MVD should be routinely detected in patients with lung adenocarcinoma after operation, and the patients with low CD34-MVD should be examined and evaluated for tumor recurrence more frequently during the follow-up period.
Antiangiogenic therapy, such as bevacizumab, can inhibit tumor angiogenesis and normalize tumor blood vessels. Hence, this is currently an important approach for antitumor drug therapy [28]. Some studies have found that antiangiogenic therapy, such as chemotherapy plus bevacizumab [22]or anlotinib alone [29], is more effective in patients with more undifferentiated (CD31+/CD34-) MVD in advanced NSCLC. CD34 is a marker of mature microvessels in differentiated tumors, and we found that a high abundance of CD34 positive microvessels reduced tumor invasion and improved patient prognosis. This suggests that increasing CD34 positive microvessels may be a novel approach to antitumor therapy.
Previous studies on the correlation between CD34-MVD and lung cancer have yielded mixed results, which may have been due to the following reasons. First, different MVD evaluation and counting methods were employed. Tanaka et al. [12], Kyuichi et al. [14] and Bing et al. [15] used the hotspot method in their studies, in which several areas with the densest concentration of microvessels were selected at low power and counted at high power. The average value was used as the final MVD value. In contrast, Donnem et al. [13] and Pomme et al. [16] used the microarray construction method in their studies, where MVD was defined as the number of microvessels found within a microarray core (0.6 mm in diameter). Second, different grouping criteria were used for MVD. Tanaka et al. [12] defined the high and low MVD groups using the median as the cutoff. The MVD values in the study by Donnem et al. [13] were based on IHC scoring. Kadota et al. [14] defined the high and low MVD groups by referring to the results of other previous studies. Pomme et al. [16] directly defined 65/mm2 as the cutoff for high and low MVD (the authors did not justify this definition). Bing et al. [15] did not divide MVD into high and low groups. Since the Weidner correction method (i.e., the hot spot method) was first proposed, it has gradually gained popularity as the most classic and widely adopted method for MVD measurement owing to its simplicity and rapidity. However, with advances in artificial intelligence and algorithms, MVD measurements assisted by computer imaging technology are expected to become a reliable indicator for evaluating therapeutic efficacy in the future [30, 31]. Based on the above, we can see that there is as yet no unified standard for MVD counting and grouping protocols, and the standardization and automation of its application should be the direction of future research.
In conclusion, our study demonstrated that a high abundance of intratumoral CD34 positive microvessels in LUAD may reduce tumor invasion, whereas a decrease in CD34 positive microvessels may be associated with tumor progression. CD34-MVD is an independent risk factor for postoperative tumor recurrence in patients with LUAD and can serve as a prognostic indicator for such patients.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the Second Affiliated Hospital of the Zhejiang University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of our previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Informed consent was waived by our ethics committee because of the retrospective nature of our study.
Author contributions
ZQ: Conceptualization, Investigation, Methodology, Writing-original draft, Writing-review and editing. JW: Conceptualization, Investigation, Methodology, Writing-original draft, Writing-review and editing. GP: Formal analysis, Data Curation, Writing-original draft, Writing-review and editing. XX: Methodology, Resources. JL: Methodology, Resources. PW: Conceptualization, Writing-original draft, Writing-review and editing, Supervision, Project administration, Funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (grant number 82371837) and the brand discipline research projects of Quzhou people’s hospital (grant number XK2022-04).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2025.1611985/full#supplementary-material
References
1.
Bray F Laversanne M Sung H Ferlay J Siegel RL Soerjomataram I et al Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2024) 68:394–424. 10.3322/caac.21492
2.
Qi J Li M Wang L Hu Y Liu W Long Z et al National and subnational trends in cancer burden in China, 2005-20: an analysis of national mortality surveillance data. Lancet Public Health (2023) 8(12):e943–e955. 10.1016/S2468-2667(23)00211-6
3.
Zhang Y Vaccarella S Morgan E Li M Etxeberria J Chokunonga E et al Global variations in lung cancer incidence by histological subtype in 2020: a population-based study. Lancet Oncol (2023) 24(11):1206–18. 10.1016/S1470-2045(23)00444-8
4.
Muraoka Y Yotsukura M Yoshida Y Nakagawa K Shiraishi K Kohno T et al Dynamics of recurrence after curative resection of nonsmall cell lung cancer. J Surg Oncol (2023) 128(7):1205–12. 10.1002/jso.27395
5.
Meira DD de Castro ECMC Casotti MC Zetum ASS Gonçalves AFM Moreira AR et al Prognostic factors and markers in non-small cell lung cancer: recent progress and future challenges. Genes (Basel) (2023) 14(10):1906. 10.3390/genes14101906
6.
Schegoleva AA Khozyainova AA Fedorov AA Gerashchenko TS Rodionov EO Topolnitsky EB et al Prognosis of different types of non-small cell lung cancer progression: current state and perspectives. Cell Physiol Biochem (2021) 55(S2):29–48. 10.33594/000000340
7.
Weis SM Cheresh DA . Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med (2011) 17(11):1359–70. 10.1038/nm.2537
8.
Nico B Benagiano V Mangieri D Maruotti N Vacca A Ribatti D . Evaluation of microvascular density in tumors: pro and contra. Histol Histopathol (2008) 23(5):601–7. 10.14670/HH-23.601
9.
Marion-Audibert AM Barel C Gouysse G Dumortier J Pilleul F Pourreyron C et al Low microvessel density is an unfavorable histoprognostic factor in pancreatic endocrine tumors. Gastroenterology (2003) 125(4):1094–104. 10.1016/s0016-5085(03)01198-3
10.
Meert AP Paesmans M Martin B Delmotte P Berghmans T Verdebout JM et al The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer (2002) 87(7):694–701. 10.1038/sj.bjc.6600551
11.
Yildiz E Ayan S Goze F Gokce G Gultekin EY . Relation of microvessel density with microvascular invasion, metastasis and prognosis in renal cell carcinoma. BJU Int (2008) 101(6):758–64. 10.1111/j.1464-410X.2007.07318.x
12.
Tanaka F Otake Y Yanagihara K Kawano Y Miyahara R Li M et al Evaluation of angiogenesis in non-small cell lung cancer: comparison between anti-CD34 antibody and anti-CD105 antibody. Clin Cancer Res (2001) 7(11):3410–5.
13.
Donnem T Al-Saad S Al-Shibli K Delghandi MP Persson M Nilsen MN et al Inverse prognostic impact of angiogenic marker expression in tumor cells versus stromal cells in non small cell lung cancer. Clin Cancer Res (2007) 13(22 Pt 1):6649–57. 10.1158/1078-0432.CCR-07-0414
14.
Kadota K Huang CL Liu D Ueno M Kushida Y Haba R et al The clinical significance of lymphangiogenesis and angiogenesis in non-small cell lung cancer patients. Eur J Cancer (2008) 44(7):1057–67. 10.1016/j.ejca.2008.03.012
15.
Bing Z Jian-ru Y Yao-quan J Shi-feng C . Evaluation of angiogenesis in non-small cell lung carcinoma by CD34 immunohistochemistry. Cell Biochem Biophys (2014) 70(1):327–31. 10.1007/s12013-014-9916-5
16.
Pomme G Augustin F Fiegl M Droeser RA Sterlacci W Tzankov A . Detailed assessment of microvasculature markers in non-small cell lung cancer reveals potentially clinically relevant characteristics. Virchows Arch (2015) 467(1):55–66. 10.1007/s00428-015-1767-y
17.
Weidner N Folkman J Pozza F Bevilacqua P Allred EN Moore DH et al Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst (1992) 84(24):1875–87. 10.1093/jnci/84.24.1875
18.
Lugano R Ramachandran M Dimberg A . Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci (2020) 77(9):1745–70. 10.1007/s00018-019-03351-7
19.
Mashreghi M Azarpara H Bazaz MR Jafari A Masoudifar A Mirzaei H et al Angiogenesis biomarkers and their targeting ligands as potential targets for tumor angiogenesis. J Cell Physiol (2018) 233(4):2949–65. 10.1002/jcp.26049
20.
Rakocevic J Orlic D Mitrovic-Ajtic O Tomasevic M Dobric M Zlatic N et al Endothelial cell markers from clinician's perspective. Exp Mol Pathol (2017) 102(2):303–13. 10.1016/j.yexmp.2017.02.005
21.
Carlini MJ Dalurzo MC Lastiri JM Smith DE Vasallo BC Puricelli LI et al Mast cell phenotypes and microvessels in non-small cell lung cancer and its prognostic significance. Hum Pathol (2010) 41(5):697–705. 10.1016/j.humpath.2009.04.029
22.
Zhao YY Xue C Jiang W Zhao HY Huang Y Feenstra K et al Predictive value of intratumoral microvascular density in patients with advanced non-small cell lung cancer receiving chemotherapy plus bevacizumab. J Thorac Oncol (2012) 7(1):71–5. 10.1097/JTO.0b013e31823085f4
23.
Yao X Qian CN Zhang ZF Tan MH Kort EJ Yang XJ et al Two distinct types of blood vessels in clear cell renal cell carcinoma have contrasting prognostic implications. Clin Cancer Res (2007) 13(1):161–9. 10.1158/1078-0432.CCR-06-0774
24.
Wang HB Qin Y Yang JY . Research on the prognosis of different types of microvessels in bladder transitional cell carcinoma. World J Clin Cases (2021) 9(25):7381–90. 10.12998/wjcc.v9.i25.7381
25.
Sopo M Anttila M Muukkonen OT YlÄ-Herttuala S Kosma VM Keski-Nisula L et al Microvessels in epithelial ovarian tumors: high microvessel density is a significant feature of malignant ovarian tumors. Anticancer Res (2020) 40(12):6923–31. 10.21873/anticanres.14716
26.
Yugawa K Itoh S Yoshizumi T Iseda N Tomiyama T Toshima T et al Prognostic impact of tumor microvessels in intrahepatic cholangiocarcinoma: association with tumor-infiltrating lymphocytes. Mod Pathol (2021) 34(4):798–807. 10.1038/s41379-020-00702-9
27.
Murakami K Kasajima A Kawagishi N Ohuchi N Sasano H . Microvessel density in hepatocellular carcinoma: prognostic significance and review of the previous published work. Hepatol Res (2015) 45(12):1185–94. 10.1111/hepr.12487
28.
Garcia J Hurwitz HI Sandler AB Miles D Coleman RL Deurloo R et al Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev (2020) 86:102017. 10.1016/j.ctrv.2020.102017
29.
Liu D Ding G . Predictive value of microvascular density for response to anlotinib in advanced NSCLC. Medicine (Baltimore) (2022) 101(3):e28647. 10.1097/MD.0000000000028647
30.
Zhang Q Wu J Bai X Liang T . Evaluation of intra-tumoral vascularization in hepatocellular carcinomas. Front Med (Lausanne) (2020) 7:584250. 10.3389/fmed.2020.584250
31.
Adamo A Bruno A Menallo G Francipane MG Fazzari M Pirrone R et al Blood vessel detection algorithm for tissue engineering and quantitative histology. Ann Biomed Eng (2022) 50(4):387–400. 10.1007/s10439-022-02923-2
Summary
Keywords
CD34, microvascular density, lung adenocarcinoma, prognosis, tumor recurrence
Citation
Qiu Z, Wu J, Pang G, Xu X, Lin J and Wang P (2025) CD34 evaluation of microvasculature in lung adenocarcinoma and its microvascular density predicts postoperative tumor recurrence. Pathol. Oncol. Res. 31:1611985. doi: 10.3389/pore.2025.1611985
Received
30 September 2024
Accepted
07 January 2025
Published
20 January 2025
Volume
31 - 2025
Edited by
Jelena Stojsic, University of Belgrade, Serbia
Updates
Copyright
© 2025 Qiu, Wu, Pang, Xu, Lin and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pingli Wang, pingliwang@zju.edu.cn
‡These authors have contributed equally to this work
ORCID: Zijian Qiu, orcid.org/0009-0007-6659-4824; Pingli Wang, orcid.org/0000-0002-1472-1242
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.