- 1Graduate School, Chengdu Medical College, Chengdu, China
- 2Department of Radiology, People’s Hospital of Lushan County, Ya’an, China
- 3Department of Pathology and Pathophysiology, Chengdu Medical College, Chengdu, China
Objectives: Summarize the progress and hot topic evolution of non-coding RNAs (ncRNAs) research in esophageal squamous cell carcinoma (ESCC) in recent years and predict future research directions.
Methods: Relevant articles from the Web of Science until 31 October 2023 were obtained. Bibliometric analysis of included articles was performed using software (VOSviewer, CiteSpace, and Bibliometrix). The volume and citation of publications, as well as the country, institution, author, journal, keywords of the articles were used as variables to analyze the research trends and hot spot evolution.
Results: 1,118 literature from 2008 to 2023 were retrieved from database, with 25 countries/regions, 793 institutions, 5,426 authors, 261 journals involved. Global cooperation was centered on China, Japan, and the United States. Zhengzhou University, an institution from China, had the highest publication. The most prolific author was Guo Wei, and the most prolific journal was Oncology Letters. Analysis of keywords revealed that the research in this field revolved around the role of ncRNAs in the occurrence, development, diagnosis, treatment, and prognosis of ESCC, mainly including micro RNAs, long non-coding RNAs, and then circular RNAs.
Conclusion: Overall, research on ncRNAs in ESCC remains strong. Previous research has mainly focused on the basic research, with a focus on the mechanism of ncRNAs in the occurrence, development, diagnosis, treatment, and prognosis of ESCC. Combining current research with emerging disciplines to further explore its mechanisms of action or shifting the focus of research from preclinical research to clinical research based on diagnosis, treatment, and prognosis, will be the main breakthrough in this field in the future.
Introduction
Esophageal squamous cell carcinoma (ESCC) is a highly aggressive malignancy with the seventh incidence rate and a leading cause of cancer-related deaths around the world [1]. Despite significant advancements in surgical techniques, chemotherapy, and radiation therapy, as well as the emergence of new targeted therapy and immunotherapy, the five-year survival rate of ESCC patients remains low at only 10%–25% [2]. Thus, the identification of novel biomarkers and therapeutic targets is essential. Non-coding RNAs (ncRNAs) constitute 98%–99% of the transcriptome, and do not encode proteins. Instead, they play crucial roles in various biological functions [3]. In recent years, ncRNAs have emerged as promising diagnostic and prognostic biomarkers, and as potential therapeutic targets in ESCC, and the most widely studied ncRNAs are microRNAs (miRNAs), long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs). These ncRNAs are abnormally expressed in ESCC and participate in various biological functions such as gene expression, cell cycle, and epithelial-mesenchymal transition (EMT) through multiple mechanisms, promoting the occurrence and development of ESCC [4–6].
Bibliometrics is a method of quantitative analysis and evaluation based on the use of literature database records. It is employed to analyze the production efficiency and characteristics of publications in a particular field, assess the quality and impact of academic outcomes, and forecast trends and directions [7, 8]. So far, bibliometric has been widely used in the fields of biosciences and clinical medicine and has provided a new horizon for research in related fields [9–11].
Currently, there has been a large amount of previous research on ncRNAs associated with ESCC worldwide, and numerous research achievements have been obtained. However, the research progress and hot spot evolution in the field are still unclear. Therefore, a comprehensive bibliometric visualization analysis of the research on ncRNAs in ESCC is warranted. This study analyzed previous studies on ncRNAs associated with ESCC using bibliometric approach and visualized the results in an effort to gain insight into the past and current research status, identify potential directions for the future research in this area.
Methods
Literature acquisition and data collection strategy
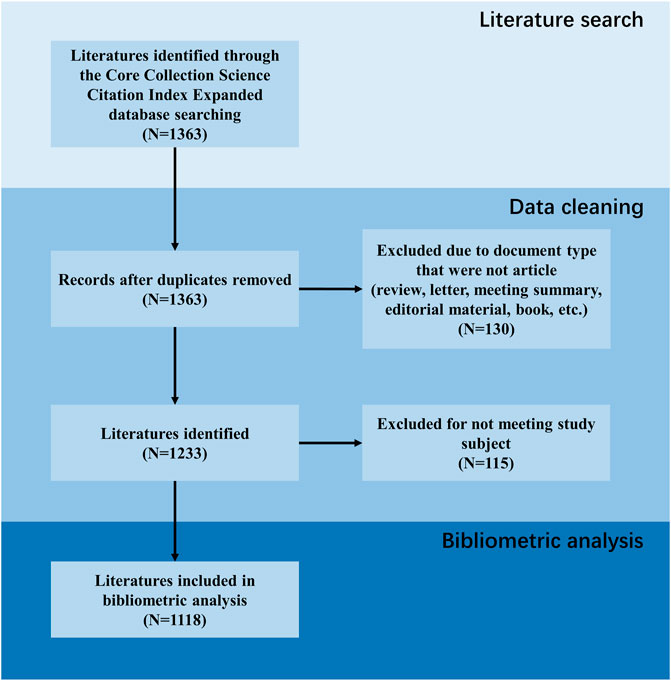
To guarantee comprehensive and accurate data retrieval, literature was searched in the Web of Science Core Collection Science Citation Index Expanded (SCIE) database until 31 October 2023 and exported as “plain text” format. Two reviewers conducted a systematic literature search independently. A senior investigator made final decisions concerning any discrepancies between the two independent reviewers. As shown in Figure 1, after obtaining literature from database, data cleaning was carried out. Duplicate documents were first excluded, followed by document type that were not articles (including review, letter, meeting summary, editorial material, book, etc.). Finally, the content of the literature was reviewed and those that did not meet the research theme were also excluded. The search formula was as follows:
#1: TS = (“esophageal squamous cell carcinoma” OR “oesophageal squamous cell carcinoma”).
#2: TS = (“non-coding RNA” OR “noncoding RNA” OR “ncRNA” OR “microRNA” OR “miRNA” OR “small RNA” OR “snoRNA” OR “snRNA” OR “long non-coding RNA” OR “lncRNA” OR “circular RNA” OR “circRNA” OR “piwi-interacting RNA” OR “piRNA”).
#3: #1 AND #2.
Bibliometric analysis and visualization
We mainly used VOSviewer (ver. 1.6.19), CiteSpace (ver. 6.1.R 2), and R package Bibliometrix (ver. 4.1.2) for the analysis and visualization of the retrieved data [12]. Annual volume of publications as well as keywords with the strongest citation burst were obtained using CiteSpace. Publication information of the source journals and high-citation articles were obtained using VOSviewer. The co-authorship analysis of country/region, organization, and author were analyzed and visualized using VOSviewer. A world map enabling visualization of the volume of publications and cooperation between countries was drawn using R package Bibliometrix. After manually standardized keywords with different spellings but same meanings, the 100 keywords with the highest frequency of occurrence were obtained using VOSviewer and visualized according to the strength of association and average year of occurrence.
Results
The annual trend in publications
We obtained literature from the SCIE database according to the developed search strategy and removed records that did not meet the inclusion criteria. The above process is presented in Figure 1, identifying a total of 1,118 articles included in this study.
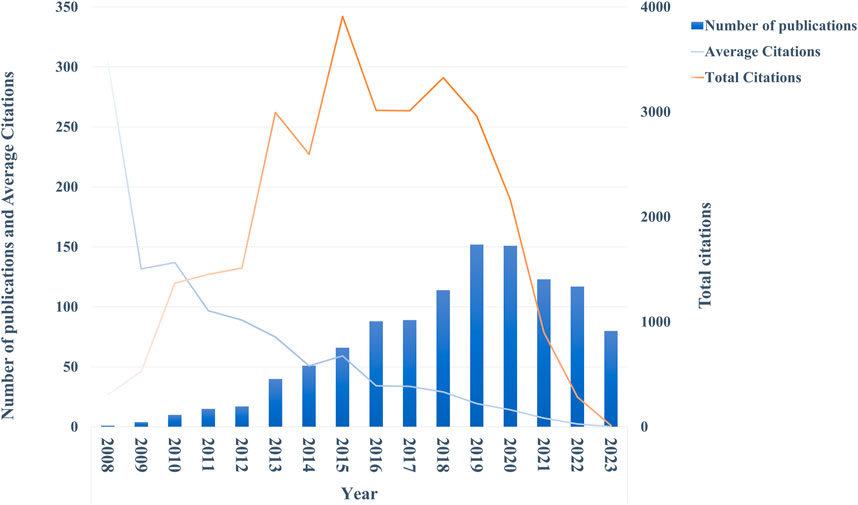
As shown in Figure 2, over the past 15 years, there had been an annual increase in publications on ncRNAs associated with ESCC. Since the first publication in 2008, the annual publications had begun to climb. In 2014, the number breached 50, and in 2018 it surpassed 100. The peak was reached in 2019 with 152 publications. However, the volume of publications declined in the following 3 years compared with 2019. In addition, the increasing trend of total citations was not as robust as the volume of publications, and it showed a decreasing trend after peaking in 2015. Notably, the mean citation of papers in the field was high at first but decreased year after year, apart from a transient rise in 2010 and 2015.
The co-authorship analysis of countries and organizations
There were 25 countries/regions that have conducted relevant research, Supplementary Table S1 lists the countries with the top 10 publications. Among them, China had the highest publications, with a total of 980 articles published, followed by Japan (N = 66) and the United States (N = 57). Countries with more than 10 publications were Iran, Australia, South Korea and Germany, the remaining countries had either 10 publications or no previous publications. As for the average citation, the highest ranking was in the order of Japan (N = 65.20), Australia (N = 49.71), South Korea (N = 38.18). To gain further insight into regional cooperation in publications, we conducted a co-authorship analysis and visualized the results. In Figure 3A, it can be seen that Asia, represented by China and Japan, and North America, represented by the United States, became the center of the current research, countries from other continents also participated in the study through direct or indirect collaboration. Figure 3B further validates this claim through a cooperation network of the world map.
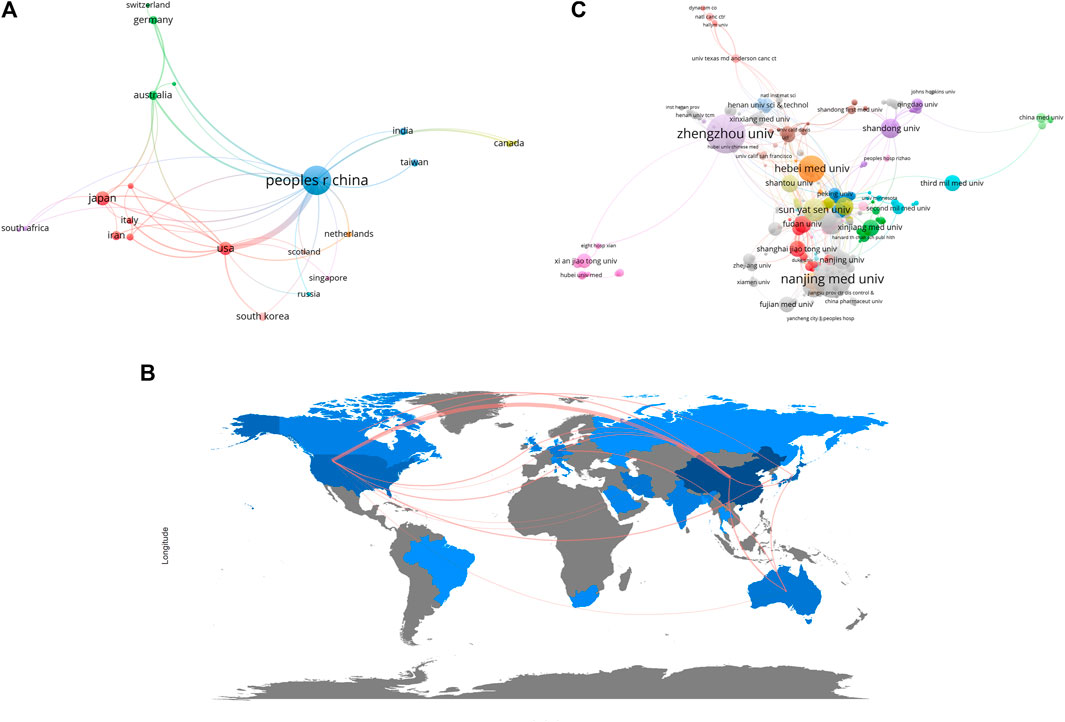
FIGURE 3. The co-authorship analysis of ncRNAs associated with ESCC. (A) Network plot of co-authorship analysis of countries/regions. (B) World map of co-authorship analysis of countries/regions. (C) Network plot of co-authorship analysis of organizations. The size of the circles or the depth of map color blocks represents the number of publications, and the thickness of the lines between the circles or the map color blocks represents the degree of collaboration.
A total of 793 organizations worldwide had participated in the field. Zhengzhou University had the largest number of publications (N = 118), followed by Nanjing Medical University (N = 110) and Hebei Medical University (N = 55). We included in Supplementary Table S2 the organizations with the top 10 publications, all of them were Chinese institutions. We performed co-authorship analysis in order to better understand publication cooperation between organizations (Figure 3C). Compared with other countries, the institutes within China were numerous and more cooperative. Among them, Zhengzhou University, Nanjing Medical University, and Hebei Medical University were the most active in cooperative research.
The co-authorship analysis of authors and co-cited authors
A total of 5,426 authors took part in the relevant research over the past 15 years. We listed the top 10 high productive authors in Supplementary Table S3. The first three scholars, all from Hebei Medical University, were Guo Wei, Dong Zhiming, Guo Yanli. It also can be found that these high-yielding scholars were all from China, suggesting the important contributions made by Chinese scholars in the current field. To reveal the contribution and cooperation of authors, the co-authorship analysis of top 100 authors was conducted (Figure 4A). By visualizing the network according to the mean publication year, two largest clusters can be found to be representative: the earlier conducted studies by the red cluster have authors Bai Yun, Li Juan, and Wang Kai, while the currently active purple cluster in the field have authors Guo Wei, Dong Zhiming, and Guo Yanli (Figure 4B).
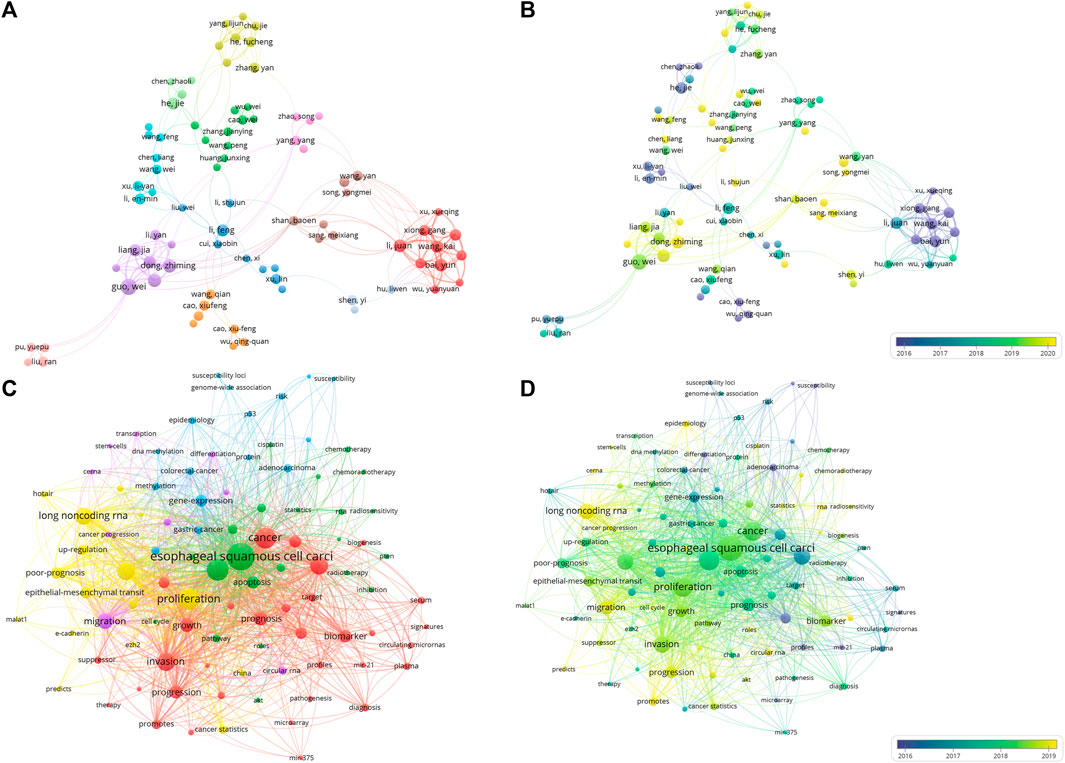
FIGURE 4. The co-authorship analysis and co-occurrence analysis of ncRNAs associated with ESCC. (A) Network plot of co-authorship analysis of authors. (B) Overlay map of co-authorship analysis of authors. (C) Network plot of co-occurrence analysis of top 100 keywords. (D) Overlay map of co-occurrence analysis of top 100 keywords. In network plot, a circle represents an author or a keyword. The larger the circle, the more articles the author has published or the more frequently the keyword appears. The thickness of the connecting line between circles represents the strength of the association. In overlay map, cold colors indicate that the author was active earlier or the keywords appeared earlier, while warm colors indicate that the author was active more recently or the keywords appeared more recently.
By analyzing co-cited authors, we learn about the impact and contribution of authors in this academia, as well as examining interdigitation and collaboration in different disciplinary areas. Supplementary Table S3 demonstrates the top 10 co-cited authors. The first three authors were David P Bartel from Howard Hughes Medical Institute (United States ), George A Calin from Ohio State University (United States ), and Chen Wanqing from National Cancer Center (China). It can be found that most of these authors were from the United States, illustrating the founder contribution that American scholars have made to the development of this field. Furthermore, the authors come from different fields, embodying that modern scientific research is the product of the intersection of different disciplines.
The distribution of source journals and highly cited articles
A total of 261 journals had published articles on ncRNAs associated with ESCC. Among them, Oncology Letters published the most article (N = 31), followed by Oncotarget (N = 30), Oncology Reports (N = 28). From the total citations, the number one was Oncotarget (N = 1958), followed by Tumor Biology (N = 719) and Oncology Reports (N = 623). Supplementary Table S4 lists the top 10 journals with the largest number of publications. Of these, one journal had an impact factor greater than 5: Biomedicine & Pharmacotherapy (IF = 7.419). Additionally, Oncotarget and International Journal of Clinical and Experimental Pathology had no impact factor because they were not currently included in the SCIE database since 2018 and 2020, respectively.
The citations of a paper can reflect its impact and visibility in the field to some extent. The total number of citations for the 1,118 articles were 30,340, with an average of 29 citations per article. The top 10 most cited articles are shown in Supplementary Table S5, with frequencies ranging from 237 to 551. These articles were published between 2008 and 2015, with the 2008 publication titled Distinctive microRNA profiles relating to patient survival in esophageal squamous cell carcinoma being the earliest in the field, the prelude to the research on ncRNAs associated with ESCC has since been revealed. Articles titled Circular RNA ITCH has inhibitory effect on ESCC by suppressing the Wnt/β-catenin pathway, published in 2015, was the most highly cited with up to 551 citations. Furthermore, these articles were authored by scholars from Japan and China, with 7 articles authored by Chinese scholars and 3 articles authored by Japanese scholars.
Keywords analysis
We conducted cluster analysis of the top 100 keywords according to the frequency of their occurrence (each keyword appeared at least 12 times). In Figure 4C, analyzing the clusters reveals that the 100 keywords are mainly divided into five clusters. The red cluster mainly includes keywords such as microRNA, biomarker, tumor-growth, prognosis and invasion, this suggests that miRNAs are potential biomarkers for ESCC, and their expression levels correlate with the occurrence and development of ESCC. Using these miRNAs as biomarkers could aid in the diagnosis and treatment of ESCC. The green cluster mainly includes ESCC, cancer cell, apoptosis, autophagy, mechanism, radiosensitivity, uncovering the cellular and molecular mechanisms by which ncRNAs participate in the development and progression of ESCC, and the association between ncRNAs and radiotherapy sensitivity of ESCC. The yellow cluster mainly includes long non-coding RNAs, metastasis and EMT, indicating the important roles and regulatory mechanisms of lncRNAs in the development and progression of ESCC. The purple cluster mainly includes migration, circular RNA and ceRNA, this suggests that circRNAs as ceRNA (competing endogenous RNA) involved in the development and progression of ESCC. The blue cluster mainly includes gene-express, DNA methylation, protein and susceptibility loci, these have implicated related molecular mechanisms in the regulation of ncRNAs in ESCC.
According to the average publication year, overlay visualization of the co-occurrence network of 100 keywords can reveal the prevalence trends of keywords. Cool colors denote earlier published keywords, whereas warm colors indicate later ones. As shown in Figure 4D, it is not difficult to find that the earliest keywords to appear were polymorphism, susceptibility, tumor-suppressor gene, profiles, mir-21, signatures, microarray, and the latest keywords were ceRNA, cancer progression, circular RNA, exosome. To explore the variation and trend of keywords in this field, we plotted a timeline for the high-frequency keywords that appear each year. As shown in Figure 5, It can be found that the early focus of this field was on the expression profile of ncRNAs, as well as the biological process of ncRNAs on the differentiation and apoptosis of ESCC cells, and on predicting the survival of ESCC patients. At this point, the main focus of attention on ncRNAs was miRNAs (2008–2012). In the mid-term stage of this field, more attention had been paid to the mechanisms by which ncRNAs participate in the occurrence and development of ESCC, including EMT, migration, etc. In addition, attention had been paid to the role of ncRNAs in the treatment and prognosis of ESCC. During this stage, lncRNAs gradually gained attention (2012–2016). At the present stage, based on the long-term research on the role of ncRNAs in the occurrence, development, diagnosis, treatment and prognosis of ESCC, circRNAs began to receive attention. And the recent keywords in this field were vegf, pi3k and hcp5 (2016–2022). In order to further understand the outbreak range of hotspots, we identified the top 25 keywords with the strongest citation burst in Figure 6. Focusing on these keywords, it can be observed that researchers have extensively concentrated on the role of ncRNAs in the development, progression, and prognosis of ESCC, along with the related mechanisms of their regulation. It can also be seen that the earliest ncRNAs that had received attention were miRNAs with the citation burst in 2010–2013, while circRNAs and lncRNAs are the current and future research trend with the citation burst in 2020–2023.
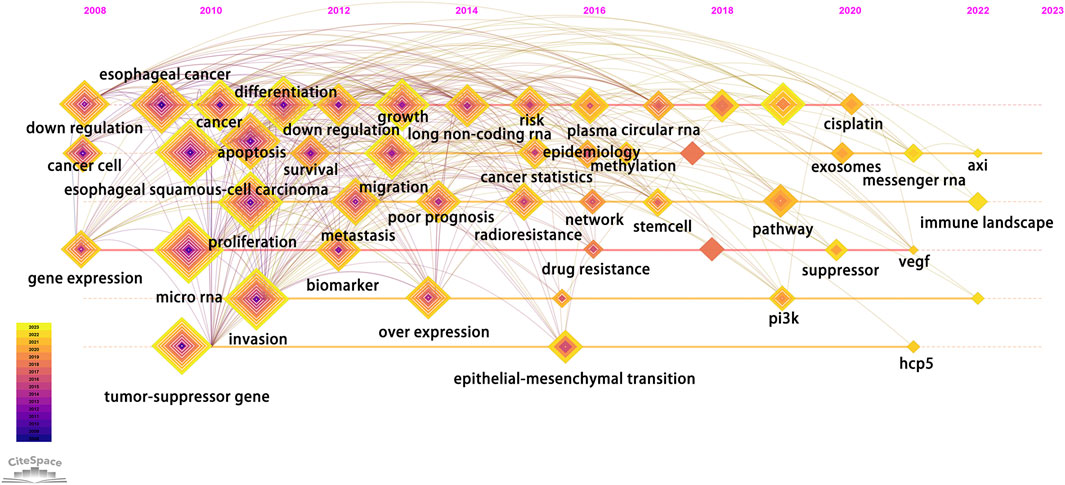
FIGURE 5. Timeline chart of keywords. Each diamond represents a keyword, and its position on the timeline represents the time it appeared. In addition, the size of the diamond represents the number of times it appears in the total time period, while each layer inside the diamond represents the number of times it appears in the corresponding year.
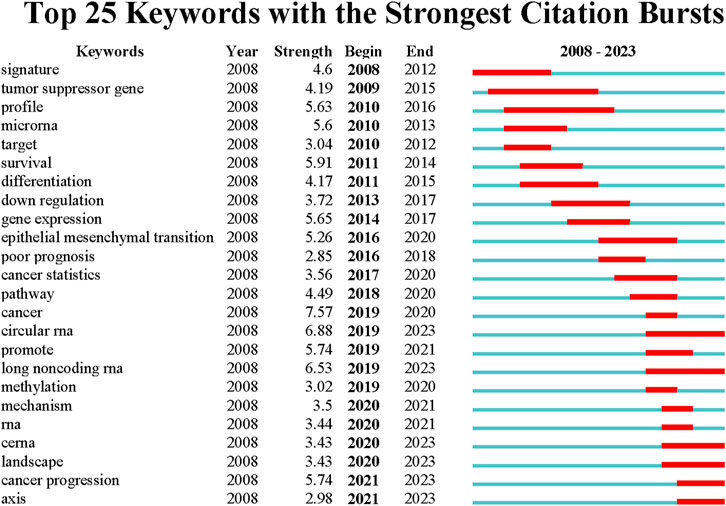
FIGURE 6. Top 25 keywords with the strongest citation burst. Blue lines indicate the time period in which the keywords appeared and ended, and red lines indicate the time period in which the keywords at high frequencies.
Discussion
Our study delved into the research of ncRNAs associated with ESCC through a bibliometric analysis that reviewed 1,118 relevant articles indexed in the SCIE database from 2008 to 2023. We scrutinized annual publication trends, affiliations, authors, co-cited authors, source journals, highly cited articles, keywords, and further investigated the current development trends and hot topics in this area.
General overview
By analyzing the trends in annual publications, the development of the research field can be divided into three phases: slow rise in the early stage (2008–2012), rapid rise stage (2013–2019), and stagnation in the later stage (2020–2023) (Figure 2). The trends in the first two stages may be attributed to the development of high-throughput sequencing technology. The article titled Distinctive microRNA profiles relating to patient survival in esophageal squamous cell carcinoma published by Guo Y et al. was the first publication in the field and one of the most highly cited articles [13] (Supplementary Table S3). The authors used advanced microRNA microarray technology to study the expression of miRNAs in esophageal cancer tissue and identified 46 RNAs that were specifically expressed in tumor tissues. Thereafter, lncRNAs and circRNAs expression profiles in ESCC were sequentially revealed [14, 15]. Building on previous work from these studies, researchers can move to deeper studies around ncRNAs and ESCC. For example, mining sequencing results through bioinformatics technology and annotating differential genes can help reveal the molecular mechanism of ncRNAs in ESCC [16, 17]. Another possible reason why research on ncRNAs in ESCC had been so popular is that ncRNAs are numerous of the transcriptome and are involved in many cellular processes [3], yet previous research in this area has been lacking. Therefore, it attracted the attention from researchers. However, the third stage of annual publications perhaps indicating that research in the field had encountered bottlenecks. The possible explanations for why there is a trend towards stagnation when it comes to investigating the mechanism of ncRNAs in ESCC could be several. Firstly, previous studies had exhaustively investigated the role of ncRNAs in ESCC, leading to the exhaustion of the field. Secondly, the research conducted so far has almost been limited to basic research, which had hindered the translation of the research results into clinical applications. Thirdly, the ncRNAs mechanism in ESCC is complex, and the field is striving to find new insights into this diverse mechanism. However, the current research model is tending towards homogenization.
In this field, China had the largest number of organizations and the most prolific authors engaged in relevant research, which had resulted in the highest productions. Japanese papers had the highest average citation rate, while the greatest number of co-cited authors cited were from the United States (Supplementary Tables S1–S3). This indicated that China was at the forefront in terms of the high yield in the field. Japanese and American research achievements in this field had a high level of influence and recognition. It can be explained by the fact that Japan and China are high-risk countries for esophageal cancer, both countries attach great importance to research on esophageal cancer [1]. And the United States holds strong strength for biomedical research [18]. In addition, the Matthew effect can also be used as a possible explanation, as these high-yielding countries have more output compared to other countries with fewer resources due to their existing research funding support or research capabilities [19]. It is worth noting that despite having the highest publication volume, China’s average citation of papers was not optimistic, indicating the need for further efforts to enhance the quality of research. As shown in Figure 3, 25 countries had participated in this research area, and a research center has been formed with China, Japan, and the United States, but the situation is that there was more frequent domestic cooperation in each respective country. The reason for this phenomenon may be that language and cultural differences between different countries may cause difficulties in communication and cooperation. On the other hand, research institutions and scientists in different countries may have varying resource and interest needs, leading to difficulties in balancing cooperation between countries. Therefore, we also look forward to closer cooperation between countries in order to produce more high-quality research.
Based on publications and citations, Oncotarget was the most influential journal in this field (Supplementary Table S4). However, the journal was not indexed by SCIE in 2018 due to excessive self-citation and potentially unjustified co-authored research. Similarly, International Journal of Clinical and Experimental Pathology was not indexed by SCIE in 2020. After excluding these two journals, Tumor Biology had the highest average citation count, establishing it as the most influential journal in this field. It is an open access journal focused on tumor related research, which also suggests that open access journals have become increasingly popular in recent years as they provide free and unrestricted access to research articles, making it easier for researchers and academics to access and share knowledge. This has helped to increase the visibility and impact of research and has also promoted greater collaboration and exchange of ideas within the academic community. Therefore, many researchers and institutions are now recognizing the value of open access publishing and are choosing to publish their research in open access journals. Highly cited articles, to some extent, also reflect hot topics of concern in the field of scientific research, and their published journals also have an undeniable influence in this field. In addition to the aforementioned journals, the articles published in the following journals rank among the top ten in terms of citation: International Journal of Cancer, Cancer, Gut, Cancer Research, Molecular Cancer, Clinical Chemistry, Journal of Biological Chemistry, and Clinical Cancer Research. These journals are positioned in fields such as oncology, molecular biology, pharmacy, and clinical medicine, reflecting the long-standing and future scientific research model of multidisciplinary cooperation.
Research hotspots
We employed co-occurrence analysis of keyword networks (Figures 4C, D) and timeline charts (Figure 5) to unveil the distribution of research focus in the field. Additionally, we analyzed keywords with the strongest citation burst to further uncover the evolving trends of research hotspots (Figure 6). Based on the above results, we can divide the research process in this field into three parts: 1. ncRNAs as a diagnostic and prognostic marker of ESCC; 2. ncRNAs participates in the occurrence and development of ESCC; 3. the therapeutic potential of ncRNAs on ESCC. The main studied ncRNAs include miRNAs, lncRNAs, and circRNAs.
One of the most promising research parts for ncRNAs in ESCC is its potential as a diagnostic and prognostic biomarker, which has been highly valued since the beginning of research in this field. Compared to normal physiological conditions, the levels of specific ncRNAs produced by cancer cells may undergo significant alterations. The detection of their expression can yield valuable biological information [20]. Furthermore, certain ncRNAs exhibit widespread stability in bodily fluids, such as blood, saliva, and urine [21]. This forms the basis for efficient, non-invasive, and cost-effective detection methods Studies have demonstrated the utility of ncRNAs as prognostic and diagnostic biomarkers for ESCC, with miRNAs being the most well-studied in this regard. For example, studies have shown that high expression of miR-1246, miR-766-3p, miR-20b-5p, and miR-1290 in the serum of ESCC patients has strong diagnostic and prognostic potential [22–25]. Similarly, lncRNAs such as HOTAIR, MALAT1, CCAT2, and PCAT-1, and circRNAs such as circ-SLC7A, circ-SMAD7, circ_000194, and circ-GSK3β, have also been identified as potential diagnostic and prognostic biomarkers for ESCC [15, 26–32]. In addition, there is a study that has taken a step further towards the identification of miRNAs (miRNA-103, miRNA-106b, miRNA-151, miRNA-17, miRNA-181a, miRNA-21, miRNA-2 and miRNA-93) with diagnostic implications in a retrospective and prospective multicenter cohort study. The results demonstrate higher specificity and sensitivity of miRNAs markers compared with traditional tumor markers including SCC Ag, CEA and CYFRA21-1, further confirming the practical significance of serum miRNAs used as diagnostic markers for early-stage ESCC [33].
Another part of research on ncRNAs in ESCC is their role in the initiation and progression of the disease. Studies have shown that ncRNAs can act as tumor promoters or suppressors in ESCC by regulating several biological processes such as proliferation, metastasis, apoptosis, cell cycle, DNA damage repair, and EMT [34–37]. Many miRNAs play a role by binding to the 3′UTR of the target gene, blocking its translation and leading to its inactivation at the mRNA level, or participating in the related pathways of G protein coupled receptors [38–40]. LncRNAs can recruit chromatin remodeling factors or inhibit transcription factors of target gene promoters to exert its effects [41]. Recently, it was found that lncRNAs can regulate the expression of related miRNAs and then participate in the occurrence and development of ESCC. For example, lncRNA FAM83A-AS1 downregulated the expression of miRNA-214, which indirectly deregulated the repression of CDC25B by miRNA-214, and thus participated in ESCC progression [42]. Similarly, circRNAs such as circRUNX1, ciRS-7, and LPAR3 function as ceRNAs of their counterpart miRNAs, contributing to the development of ESCC [4, 43, 44]. The term “ceRNA” refers to the ability of circRNAs to serve as miRNA sponges by sequestering them and inhibiting their regulatory effects on downstream targets, thus exerting their own regulatory roles.
The third part of research on ncRNAs in ESCC is their potential for use in treatment. NcRNAs may be able to improve the efficacy of existing therapies, such as chemotherapy and radiotherapy, or act as novel therapeutic targets. The main chemotherapy regimen for ESCC patients mainly includes cis-diamminedichloro-platinum (CDDP), 5-fluorouracil, and gefitinib. If the patient is not sensitive to these anticancer drugs, it may lead to unsatisfactory treatment effectiveness [45–47]. Studies have shown that high-risk ncRNAs, such as miR-624, miR-140-3P, lncRNA NORAD, lncRNA CASC8, and hsa_circ_0000277, can increase chemotherapy resistance in ESCC patients. Therefore, reversing the expression of high-risk ncRNAs may have a positive effect on reducing chemotherapy resistance, thereby improving treatment efficacy [5, 6, 48–50]. For instance, re-expression of circRNA-cdopey2 decreased the amount of anti-apoptotic protein Mcl-1 expression, which in turn enhanced the apoptosis of ESCC cells and enhanced the killing ability of CDDP on cancer cells [51]. Jin et al. showed that the overexpression of miR-141-3p can promote acquired chemotherapy resistance of 5-fluorouracil by regulating tumor suppressor PTEN [46]. In addition, lncRNAs (such as AFAP1-AS1 and LINC01014) participate in Wnt/β-Catenin and PI3K/Akt/mTOR signaling pathways reduced the effectiveness of chemotherapy drugs including gefitinib [52]. Radiotherapy is a radical treatment modality for ESCC, but studies have found that ncRNAs are involved in the sensitivity of ESCC cells to radiation. Dysregulation of ncRNAs such as miRNA-4443 and miRNA-21 can affect cell cycle, apoptosis, and DNA damage repair, making them potential targets for improving ESCC radiosensitivity [37, 53]. The abundance of miRNA-21 can be decreased by upregulating lncRNA gas5, which further upregulates expression of a transformation repressor named RECK to enhance ESCC cell radiosensitivity [53]. Similarly, circvrk1 was found to be under expressed in ESCC tissues and cell lines compared with controls. Radiation resistance of ESCC cells could be alleviated by overexpression of circvrk1, the mechanism is that circvrk1, as the ceRNA of mir-624-3p, upregulates PTEN and exhibits a role in reducing radiation resistance [54]. In summary, ncRNAs have great potential in the field of ESCC treatment. Unfortunately, as of now, there are no large-scale, high-quality randomized controlled clinical studies to further validate.
With the emergence of new technologies, research on ncRNAs in ESCC has been further explored based on previous research models. For example, relevant research focuses on ncRNAs in exosomes, because as a subgroup of tumor microenvironment, it promotes cell communication by transmitting various biological molecules [55]. Exosomes carrying ncRNAs, due to their stability and widespread presence in body fluids, along with advancements in exosome separation technology, are expected to make the detection of ncRNAs for screening ESCC patients non-invasive, convenient, and efficient [56]. In addition, studies have also focused on the immune landscape related to ncRNAs in ESCC, which will be of great significance in revealing the tumor immune microenvironment. The continuous evolution of research on the immune landscape has given rise to a variety of novel technologies, providing more in-depth and comprehensive insights. These encompass single-cell RNA sequencing, bioinformatics, and machine learning. The integrated application of these emerging technologies enables researchers to gain a more comprehensive and profound understanding of the intricacies of the immune landscape, particularly in relation to ncRNAs [57, 58].
Therefore, it can be said that the study of ncRNAs in ESCC has opened up new avenues for the development of novel diagnostic, prognostic, and therapeutic approaches. Our study can also provide insights into the focus of future studies. On the one hand, future studies can further apply new technologies (e.g., Mendelian randomization and network pharmacology) to explore the role of ncRNAs in ESCC based on current research model. On the other hand, more in-depth studies, especially clinical studies are needed to validate the value of ncRNAs in the practical clinical application of ESCC.
Limitation
Our study utilized bibliometrics to comprehensively analyze the progression of research on ncRNAs associated with ESCC. However, we must be aware of potential limitations. Firstly, considering the completeness and authenticity of the data obtained, we used only the SCIE database to retrieve related articles. Secondly, quality assessment was not conducted due to the large volume of included literature, potentially leading to variations in article quality. Thirdly, due to the unavoidable issue of inconsistent spelling or identical names among authors, the author names automatically extracted by software may differ slightly from the actual situation, and currently, there is no effective method for bulk correction.
Conclusion
In summary, exploring the roles and mechanisms of ncRNAs in ESCC has paved the way for the discovery of novel diagnostic, prognostic, and therapeutic approaches. However, the current research paradigm in this field tends towards homogeneity. Future investigations should focus on providing more nuanced explanations for the functions of ncRNAs, aiming to gain a deeper understanding of its role in ESCC. This involves a concerted effort to translate these findings into clinical research, validating the specificity, sensitivity, and clinical feasibility of ncRNAs in ESCC. Integrating novel interdisciplinary elements into the existing research framework or shifting the research emphasis from preclinical studies to clinical investigations centered on diagnosis, treatment, and prognosis will likely constitute the primary avenues for breakthroughs in this field.
Data availability statement
The data used in this study comes from published studies, and all data related to the research results are included in the main text. For more information, please consult the corresponding authors.
Author contributions
JW was responsible for the concept and design of the project, as well as the drafting of the manuscript. YW and YC were involved in the acquisition, analysis, and interpretation of the data. LC and LZ provided administrative, technical, and material support throughout the project, and also supervised the overall process. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Project of Chengdu Medical College Graduate Innovative Scientific Research Foundation (Grant Nos. YCX2022-01-02 and YCX2023-02-06).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Jingxin Yan provided inspiration to this paper.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2024.1611595/full#supplementary-material
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer J clinicians (2021) 71(3):209–49. doi:10.3322/caac.21660
2. Huang, J, Koulaouzidis, A, Marlicz, W, Lok, V, Chu, C, Ngai, CH, et al. Global burden, risk factors, and trends of esophageal cancer: an analysis of cancer registries from 48 countries. Cancers (2021) 13(1):141. doi:10.3390/cancers13010141
3. Moore, JE, Purcaro, MJ, Pratt, HE, Epstein, CB, Shoresh, N, Adrian, J, et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature (2020) 583(7818):699–710. doi:10.1038/s41586-020-2493-4
4. Wang, C, Zhou, M, Zhu, P, Ju, C, Sheng, J, Du, D, et al. IGF2BP2-induced circRUNX1 facilitates the growth and metastasis of esophageal squamous cell carcinoma through miR-449b-5p/FOXP3 axis. J Exp Clin Cancer Res : CR (2022) 41(1):347. doi:10.1186/s13046-022-02550-8
5. Wu, Q, Zhang, H, Yang, D, Min, Q, Wang, Y, Zhang, W, et al. The m6A-induced lncRNA CASC8 promotes proliferation and chemoresistance via upregulation of hnRNPL in esophageal squamous cell carcinoma. Int J Biol Sci (2022) 18(13):4824–36. doi:10.7150/ijbs.71234
6. Song, B, Liu, X, Dong, H, and Roy, R. miR-140-3P induces chemotherapy resistance in esophageal carcinoma by targeting the NFYA-MDR1 Axis. Appl Biochem Biotechnol (2023) 195(2):973–91. doi:10.1007/s12010-022-04139-5
7. Ninkov, A, Frank, JR, and Maggio, LA. Bibliometrics: methods for studying academic publishing. Perspect Med Educ (2022) 11(3):173–6. doi:10.1007/s40037-021-00695-4
8. Okubo, Y. “Bibliometric indicators and analysis of research systems: methods and examples,” in OECD science, technology and industry working papers, No. 1997/01. Paris: OECD Publishing (1997). doi:10.1787/208277770603
9. Ge, Y, Chao, T, Sun, J, Liu, W, Chen, Y, and Wang, C. Frontiers and hotspots evolution in psycho-cardiology: a bibliometric analysis from 2004 to 2022. Curr Probl Cardiol (2022) 47(12):101361. doi:10.1016/j.cpcardiol.2022.101361
10. Ahmad, P, and Slots, J. A bibliometric analysis of periodontology. Periodontol 2000 (2021) 85(1):237–40. doi:10.1111/prd.12376
11. Hassan, W, Zafar, M, Duarte, AE, Kamdem, JP, and Teixeira da Rocha, JB. Pharmacological Research: a bibliometric analysis from 1989 to 2019. Pharmacol Res (2021) 169:105645. doi:10.1016/j.phrs.2021.105645
12. Aria, M, and Cuccurullo, C. bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetrics (2017) 11(4):959–75. doi:10.1016/j.joi.2017.08.007
13. Guo, Y, Chen, Z, Zhang, L, Zhou, F, Shi, S, Feng, X, et al. Distinctive microRNA profiles relating to patient survival in esophageal squamous cell carcinoma. Cancer Res (2008) 68(1):26–33. doi:10.1158/0008-5472.CAN-06-4418
14. Li, CQ, Huang, GW, Wu, ZY, Xu, YJ, Li, XC, Xue, YJ, et al. Integrative analyses of transcriptome sequencing identify novel functional lncRNAs in esophageal squamous cell carcinoma. Oncogenesis (2017) 6(2):e297. doi:10.1038/oncsis.2017.1
15. Fan, L, Cao, Q, Liu, J, Zhang, J, and Li, B. Circular RNA profiling and its potential for esophageal squamous cell cancer diagnosis and prognosis. Mol Cancer (2019) 18(1):16. doi:10.1186/s12943-018-0936-4
16. Su, H, Lin, F, Deng, X, Shen, L, Fang, Y, Fei, Z, et al. Profiling and bioinformatics analyses reveal differential circular RNA expression in radioresistant esophageal cancer cells. J translational Med (2016) 14(1):225. doi:10.1186/s12967-016-0977-7
17. Liu, H, Zhang, Q, Lou, Q, Zhang, X, Cui, Y, Wang, P, et al. Differential analysis of lncRNA, miRNA and mRNA expression profiles and the prognostic value of lncRNA in esophageal cancer. Pathol Oncol Res : POR (2020) 26(2):1029–39. doi:10.1007/s12253-019-00655-8
18. Wagner, CS, and Jonkers, K. Open countries have strong science. Nature (2017) 550(7674):32–3. doi:10.1038/550032a
19. Bol, T, de Vaan, M, and van de Rijt, A. The Matthew effect in science funding. Proc Natl Acad Sci USA (2018) 115(19):4887–90. doi:10.1073/pnas.1719557115
20. Zhou, H, Hao, X, Zhang, P, and He, S. Noncoding RNA mutations in cancer. Wiley Interdiscip Rev RNA (2023) 14(6):e1812. doi:10.1002/wrna.1812
21. Yan, H, and Bu, P. Non-coding RNA in cancer. Essays Biochem (2021) 65(4):625–39. doi:10.1042/EBC20200032
22. Takeshita, N, Hoshino, I, Mori, M, Akutsu, Y, Hanari, N, Yoneyama, Y, et al. Serum microRNA expression profile: miR-1246 as a novel diagnostic and prognostic biomarker for oesophageal squamous cell carcinoma. Br J Cancer (2013) 108(3):644–52. doi:10.1038/bjc.2013.8
23. Liu, S, Lin, Z, Zheng, Z, Rao, W, Lin, Y, Chen, H, et al. Serum exosomal microRNA-766-3p expression is associated with poor prognosis of esophageal squamous cell carcinoma. Cancer Sci (2020) 111(10):3881–92. doi:10.1111/cas.14550
24. Huang, Z, Zhang, L, Zhu, D, Shan, X, Zhou, X, Qi, LW, et al. A novel serum microRNA signature to screen esophageal squamous cell carcinoma. Cancer Med (2017) 6(1):109–19. doi:10.1002/cam4.973
25. Sun, H, Wang, L, Zhao, Q, and Dai, J. Diagnostic and prognostic value of serum miRNA-1290 in human esophageal squamous cell carcinoma. Cancer biomarkers : section A Dis markers (2019) 25(4):381–7. doi:10.3233/CBM-190007
26. Chen, FJ, Sun, M, Li, SQ, Wu, QQ, Ji, L, Liu, ZL, et al. Upregulation of the long non-coding RNA HOTAIR promotes esophageal squamous cell carcinoma metastasis and poor prognosis. Mol carcinogenesis (2013) 52(11):908–15. doi:10.1002/mc.21944
27. Cao, X, Zhao, R, Chen, Q, Zhao, Y, Zhang, B, Zhang, Y, et al. MALAT1 might be a predictive marker of poor prognosis in patients who underwent radical resection of middle thoracic esophageal squamous cell carcinoma. Cancer biomarkers : section A Dis markers (2015) 15(6):717–23. doi:10.3233/CBM-150513
28. Zhang, X, Xu, Y, He, C, Guo, X, Zhang, J, He, C, et al. Elevated expression of CCAT2 is associated with poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol (2015) 111(7):834–9. doi:10.1002/jso.23888
29. Shi, WH, Wu, QQ, Li, SQ, Yang, TX, Liu, ZH, Tong, YS, et al. Upregulation of the long noncoding RNA PCAT-1 correlates with advanced clinical stage and poor prognosis in esophageal squamous carcinoma. Tumour Biol : J Int Soc Oncodevelopmental Biol Med (2015) 36(4):2501–7. doi:10.1007/s13277-014-2863-3
30. Huang, E, Fu, J, Yu, Q, Xie, P, Yang, Z, Ji, H, et al. CircRNA hsa_circ_0004771 promotes esophageal squamous cell cancer progression via miR-339-5p/CDC25A axis. Epigenomics (2020) 12(7):587–603. doi:10.2217/epi-2019-0404
31. Zhang, Y, Wang, Q, Zhu, D, Rong, J, Shi, W, and Cao, X. Up-regulation of circ-SMAD7 inhibits tumor proliferation and migration in esophageal squamous cell carcinoma. Biomed Pharmacother (2019) 111:596–601. doi:10.1016/j.biopha.2018.12.116
32. Hu, X, Wu, D, He, X, Zhao, H, He, Z, Lin, J, et al. circGSK3β promotes metastasis in esophageal squamous cell carcinoma by augmenting β-catenin signaling. Mol Cancer (2019) 18(1):160. doi:10.1186/s12943-019-1095-y
33. Miyoshi, J, Zhu, Z, Luo, A, Toden, S, Zhou, X, Izumi, D, et al. A microRNA-based liquid biopsy signature for the early detection of esophageal squamous cell carcinoma: a retrospective, prospective and multicenter study. Mol Cancer (2022) 21(1):44. doi:10.1186/s12943-022-01507-x
34. Qiao, G, Dai, C, He, Y, Shi, J, and Xu, C. Effects of miR-106b-3p on cell proliferation and epithelial-mesenchymal transition, and targeting of ZNRF3 in esophageal squamous cell carcinoma. Int J Mol Med (2019) 43(4):1817–29. doi:10.3892/ijmm.2019.4107
35. Guo, Y, Sun, P, Guo, W, Yin, Q, Han, J, Sheng, S, et al. LncRNA DDX11 antisense RNA 1 promotes EMT process of esophageal squamous cell carcinoma by sponging miR-30d-5p to regulate SNAI1/ZEB2 expression and Wnt/β-catenin pathway. Bioengineered (2021) 12(2):11425–40. doi:10.1080/21655979.2021.2008759
36. Gu, L, Sang, Y, Nan, X, Zheng, Y, Liu, F, Meng, L, et al. circCYP24A1 facilitates esophageal squamous cell carcinoma progression through binding PKM2 to regulate NF-κB-induced CCL5 secretion. Mol Cancer (2022) 21(1):217. doi:10.1186/s12943-022-01686-7
37. Shi, X, Liu, X, Huang, S, Hao, Y, Pan, S, Ke, Y, et al. miR-4443 promotes radiation resistance of esophageal squamous cell carcinoma via targeting PTPRJ. J translational Med (2022) 20(1):626. doi:10.1186/s12967-022-03818-5
38. Lu, HB. MicroRNA-556-3p promotes the progression of esophageal cancer via targeting DAB2IP. Eur Rev Med Pharmacol Sci (2018) 22(20):6816–23. doi:10.26355/eurrev_201810_16149
39. Tan, X, Ren, S, Fu, MZ, Ren, S, Yang, C, Wu, X, et al. microRNA-196b promotes esophageal squamous cell carcinogenesis and chemoradioresistance by inhibiting EPHA7, thereby restoring EPHA2 activity. Am J Cancer Res (2021) 11(7):3594–610.
40. Xu, C, Li, B, Zhao, S, Jin, B, Jia, R, Ge, J, et al. MicroRNA-186-5p inhibits proliferation and metastasis of esophageal cancer by mediating HOXA9. OncoTargets Ther (2019) 12:8905–14. doi:10.2147/OTT.S227920
41. Wang, N, Yu, Y, Xu, B, Zhang, M, Li, Q, and Miao, L. Pivotal prognostic and diagnostic role of the long non-coding RNA colon cancer-associated transcript 1 expression in human cancer (Review). Mol Med Rep (2019) 19(2):771–82. doi:10.3892/mmr.2018.9721
42. Jia, J, Li, H, Chu, J, Sheng, J, Wang, C, Jia, Z, et al. LncRNA FAM83A-AS1 promotes ESCC progression by regulating miR-214/CDC25B axis. J Cancer (2021) 12(4):1200–11. doi:10.7150/jca.54007
43. Sang, M, Meng, L, Sang, Y, Liu, S, Ding, P, Ju, Y, et al. Circular RNA ciRS-7 accelerates ESCC progression through acting as a miR-876-5p sponge to enhance MAGE-A family expression. Cancer Lett (2018) 426:37–46. doi:10.1016/j.canlet.2018.03.049
44. Shi, Y, Fang, N, Li, Y, Guo, Z, Jiang, W, He, Y, et al. Circular RNA LPAR3 sponges microRNA-198 to facilitate esophageal cancer migration, invasion, and metastasis. Cancer Sci (2020) 111(8):2824–36. doi:10.1111/cas.14511
45. Toshimitsu, H, Hashimoto, K, Tangoku, A, Iizuka, N, Yamamoto, K, Kawauchi, S, et al. Molecular signature linked to acquired resistance to cisplatin in esophageal cancer cells. Cancer Lett (2004) 211(1):69–78. doi:10.1016/j.canlet.2004.01.038
46. Jin, YY, Chen, QJ, Xu, K, Ren, HT, Bao, X, Ma, YN, et al. Involvement of microRNA-141-3p in 5-fluorouracil and oxaliplatin chemo-resistance in esophageal cancer cells via regulation of PTEN. Mol Cell Biochem (2016) 422(1-2):161–70. doi:10.1007/s11010-016-2816-9
47. Zhu, H, Du, F, and Cao, C. Restoration of circPSMC3 sensitizes gefitinib-resistant esophageal squamous cell carcinoma cells to gefitinib by regulating miR-10a-5p/PTEN axis. Cel Biol Int (2021) 45(1):107–16. doi:10.1002/cbin.11473
48. Jia, Y, Tian, C, Wang, H, Yu, F, Lv, W, Duan, Y, et al. Long non-coding RNA NORAD/miR-224-3p/MTDH axis contributes to CDDP resistance of esophageal squamous cell carcinoma by promoting nuclear accumulation of β-catenin. Mol Cancer (2021) 20(1):162. doi:10.1186/s12943-021-01455-y
49. Yan, J, Shi, L, Lin, S, and Li, Y. MicroRNA-624-mediated ARRDC3/YAP/HIF1α axis enhances esophageal squamous cell carcinoma cell resistance to cisplatin and paclitaxel. Bioengineered (2021) 12(1):5334–47. doi:10.1080/21655979.2021.1938497
50. Cheng, J, Zhang, R, Yan, M, and Li, Y. Circular RNA hsa_circ_0000277 promotes tumor progression and DDP resistance in esophageal squamous cell carcinoma. BMC cancer (2022) 22(1):238. doi:10.1186/s12885-022-09241-9
51. Liu, Z, Gu, S, Wu, K, Li, L, Dong, C, Wang, W, et al. CircRNA-DOPEY2 enhances the chemosensitivity of esophageal cancer cells by inhibiting CPEB4-mediated Mcl-1 translation. J Exp Clin Cancer Res : CR (2021) 40(1):361. doi:10.1186/s13046-021-02149-5
52. Sharma, U, Murmu, M, Barwal, TS, Tuli, HS, Jain, M, Prakash, H, et al. A pleiotropic role of long non-coding RNAs in the modulation of wnt/β-catenin and PI3K/Akt/mTOR signaling pathways in esophageal squamous cell carcinoma: implication in chemotherapeutic drug response. Curr Oncol (Toronto, Ont) (2022) 29(4):2326–49. doi:10.3390/curroncol29040189
53. Lin, J, Liu, Z, Liao, S, Li, E, Wu, X, and Zeng, W. Elevation of long non-coding RNA GAS5 and knockdown of microRNA-21 up-regulate RECK expression to enhance esophageal squamous cell carcinoma cell radio-sensitivity after radiotherapy. Genomics (2020) 112(3):2173–85. doi:10.1016/j.ygeno.2019.12.013
54. He, Y, Mingyan, E, Wang, C, Liu, G, Shi, M, and Liu, S. CircVRK1 regulates tumor progression and radioresistance in esophageal squamous cell carcinoma by regulating miR-624-3p/PTEN/PI3K/AKT signaling pathway. Int J Biol macromolecules (2019) 125:116–23. doi:10.1016/j.ijbiomac.2018.11.273
55. Yao, W, Jia, X, Zhu, L, Xu, L, Zhang, Q, Xia, T, et al. Exosomal circ_0026611 contributes to lymphangiogenesis by reducing PROX1 acetylation and ubiquitination in human lymphatic endothelial cells (HLECs). Cell Mol Biol Lett (2023) 28(1):13. doi:10.1186/s11658-022-00410-z
56. Zhao, L, Yu, L, Wang, X, He, J, Zhu, X, Zhang, R, et al. Mechanisms of function and clinical potential of exosomes in esophageal squamous cell carcinoma. Cancer Lett (2023) 553:215993. doi:10.1016/j.canlet.2022.215993
57. Zhao, F, Li, Z, Dong, Z, Wang, Z, Guo, P, Zhang, D, et al. Exploring the potential of exosome-related LncRNA pairs as predictors for immune microenvironment, survival outcome, and microbiotain landscape in esophageal squamous cell carcinoma. Front Immunol (2022) 13:918154. doi:10.3389/fimmu.2022.918154
Keywords: novel biomarker, non-coding RNAs, digestive system cancer, bibliometric analysis, VOSviewer
Citation: Wu J, Wang Y, Cheng Y, Cheng L and Zhang L (2024) Comprehensive landscape and future perspectives of non-coding RNAs in esophageal squamous cell carcinoma, a bibliometric analysis from 2008 to 2023. Pathol. Oncol. Res. 30:1611595. doi: 10.3389/pore.2024.1611595
Received: 18 November 2023; Accepted: 07 February 2024;
Published: 21 February 2024.
Edited by:
Andrea Ladányi, National Institute of Oncology (NIO), HungaryCopyright © 2024 Wu, Wang, Cheng, Cheng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lushun Zhang, emhhbmdsczIwMTJAY21jLmVkdS5jbg==; Li Cheng, ODUyNjQ5NDY0QHFxLmNvbQ==
‡ORCID: Lushun Zhang, orcid.org/0000-0001-6094-2460
†These authors have contributed equally to this work
 Jiaxin Wu1†
Jiaxin Wu1† Lushun Zhang
Lushun Zhang