Abstract
Background: Waldenström macroglobulinemia (WM) is a rare subtype of B-cell lymphoma. Rituximab-based combination therapy and Bruton’s tyrosine kinase (BTK) inhibitors have greatly improved the prognosis of WM. Despite the high response rate and good tolerance of BTK inhibitors in treatment of WM, a proportion of patients still experience disease progression.
Case presentation: We report a 55-year-old man with relapsed WM. The patient achieved partial remission after six courses of CHOP chemotherapy and multiple plasma exchanges in initial treatment. He was admitted to the hospital with abdominal distension, and was diagnosed with relapsed WM and subsequently started on zanubrutinib. Disease progression and histological transformation occurred during treatment. We performed liquid biopsies on transformed plasma, tumor tissue and ascites at the same time and found high consistency between ascites and tissues. Moreover, we detected resistance mutations of BTK inhibitors (BTK, PLCG2) in ascites that were not detected in plasma or tissue. Eventually, the patient died during the 15-month follow-up after relapse.
Conclusion: We describe a rare case of WM transformation to DLCBCL treated with chemoimmunotherapy and BTK inhibition. We analyzed tumor DNA obtained at different anatomic sites and circulating tumor DNA (ctDNA) derived from plasma and ascites specimens, with apparent significant temporal and spatial heterogeneity. The case specifically highlights the clinical value of ctDNA of ascites supernatant from WM patients, which is a more convenient and relatively noninvasive method compared with traditional invasive tissue biopsy.
Introduction
Waldenström macroglobulinemia (WM), a rare form of non-Hodgkin lymphoma, is a lymphoproliferative disease featuring bone marrow infiltration and IgM monoclonal gammopathy (1). WM represents 1%–2% of all hematological malignancies, and the morbidity is approximately five cases per million person-years. There are several clinical manifestations of this disease, including anemia, thrombocytopenia, hepatosplenomegaly, lymphadenopathy, and hyperviscosity (2). The somatic mutation MYD88L265P and mutations in CXCR4 are mainly involved in the development of WM and play a crucial role in clinical features and overall survival (3). In general, updating of MYD88 and CXCR4 mutation profiling in WM has deepened the understanding of signaling pathways and promoted the development of BTK inhibitors. The 10th International Workshop for Waldenström’s macroglobulinemia (IWWM-10) recommended BTK inhibitors alone or in combination with rituximab as first-line treatment options for WM patients (4). With the advance of precision medicine, circulating tumor DNA (ctDNA) extracted from body fluids, including blood, cerebrospinal fluid, pleural effusion, and ascites, has been used for cancer genomic profiling (5–8). Resistance to BTK inhibitors secondary to histological transformation in WM is rare. Herein, we describe a patient with recurrent WM who developed histological transformation during treatment with zanubrutinib. We performed genetic testing of his pretransformed tissue and transformed ascites, plasma, and transformed tissue, and found a high degree of concordance between the ascites and transformed tumor tissue samples. This finding suggests that ascites may be used for liquid biopsy in WM to detect genetic mutations and early identification of clinical drug resistance.
Case presentation
A 55-year-old Asian man with a history of chronic hepatitis B visited the hospital in September 2013 due to recurrent dizziness, fatigue, pyrexia and night sweats for approximately 2 months. Clinical examination showed superficial lymphadenopathy and splenomegaly, with no tenderness. Laboratory examination results were as follows: hemoglobin, 40 g/L (reference range, 110–160 g/L); platelets, 130×109/L (reference range, 100–300 × 109/L); serum β-2 microglobulin, 3.85 mg/L (reference range, ≤2.80 mg/L); IgM at 67.7 g/L; (reference range, 0.4–3.0 g/L); Coombs’ test (+). An IgM-kappa monoclonal peak was observed by serum immunofixation electrophoresis. Bone marrow biopsy showed diffuse trabecular space invasion by small lymphocytes differentiated by plasma cells and confirmed the diagnosis of WM. The international prognostic scoring system for the Waldenström macroglobulinemia (IPSS WM) score was 2, indicating intermediate risk. In view of the anemia, extremely elevated IgM and hyperviscosity present, the patient received six courses of CHOP chemotherapy (R-CHOP was used once but was discontinued due to allergy) and multiple plasma exchanges. He achieved partial remission after treatment with the level of IgM falling to 30.2 g/L from November 2013 to May 2017. However, the patient did not regularly attend follow-up in the next year, even though he had recurrent abdominal discomfort. He developed increasing abdominal distention and was admitted to our department in August 2020. Physical examination revealed emaciation, anemic appearance, multiple enlarged lymph nodes in the cervical, axillary and inguinal areas, frog belly, splenomegaly, shifting dullness (+), fluid thrill and edema of the bilateral lower limbs. Laboratory examinations showed WBC at 2.83 × 109/L, hemoglobin at 85 g/L, platelets at 94 × 109/L, serum β-2 microglobulin at 6.68 mg/L, and IgM at 74.32 g/L. An IgM-kappa monoclonal peak was still observed by serum immunofixation electrophoresis. Bone marrow biopsy showed that tumor cells composed of small lymphoid cells, plasmacytoid lymphocytes and plasma cells had infiltrated the bone marrow. The bone marrow flow cytometry results were as follows: CD19+, CD20+, CD79B+, CD38+, CD138−, CD5−, CD10−, CD103−, Kappa+, Lambda−, CD200−, CD43−, FMC7p+, and CD23p+ (Figure 1). Immunohistochemical (IHC) analysis of the left inguinal lymph node biopsy showed that the tumor cells were positive for CD20, CD19, CD38, CD138, and IgM and negative for CD5, CD10, kappa, lambda and p53, which was consistent with the diagnosis of WM, with Ki-67 = 15% (Figure 2). Given the recurrence of the disease, the patient was treated with 160 mg zanubrutinib twice daily, continuous intraperitoneal drainage and albumin infusion. During the 4 months after administration of zanubrutinib, the patient experienced three infections (Escherichia coli septicemia once and left femoral periostitis two times). His hemoglobin level returned to 126 g/L, and his IgM level dropped to 26.4 g/L, with shrinkage of the spleen and retroperitoneal lymph nodes after treatment, thus achieving partial remission in December 2020 (Figure 3).
FIGURE 1
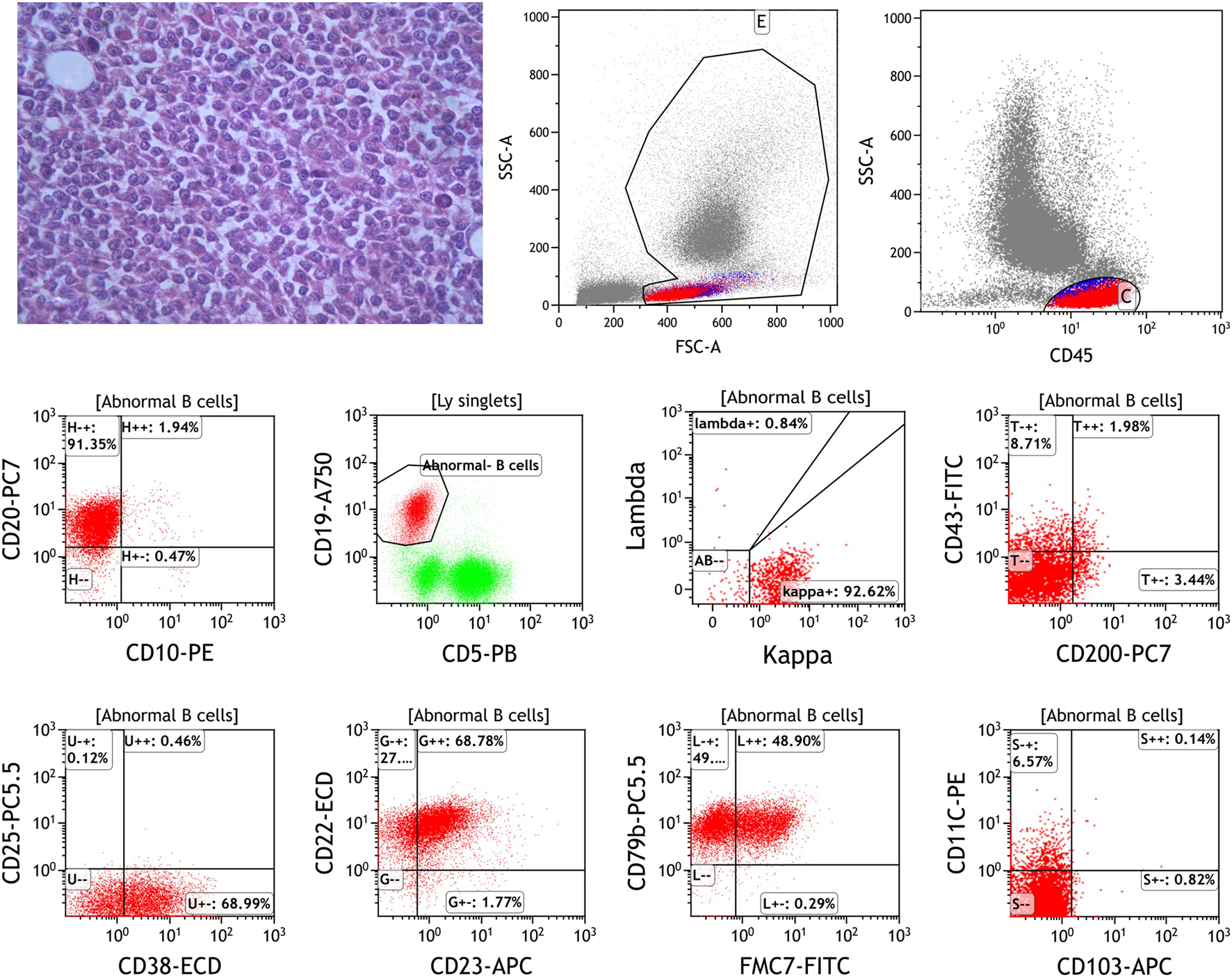
The bone marrow biopsy of the first disease recurrence showed the tumor cells composed of small lymphoid cells, plasmacytoid lymphocytes and plasma cells infiltrated the bone marrow (HEX400). Flow cytometric analysis of bone marrow specimen shows: CD20+, CD19+, CD5−, CD10−, Kappa+, Lambda−, CD200−, CD43−, CD38+, CD23p+, CD79B+, FMC7p+ and CD103−.
FIGURE 2
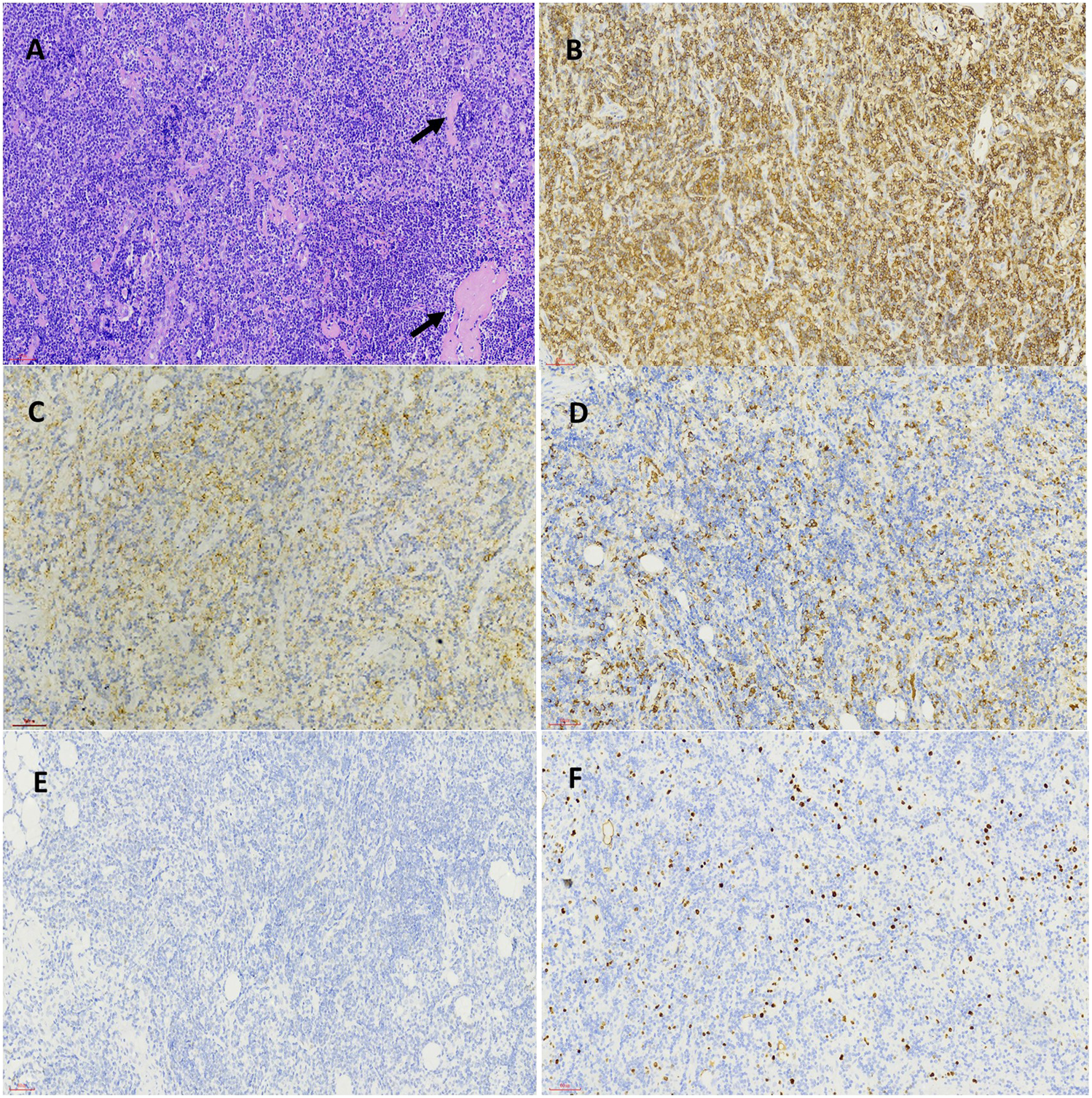
Pathological features of inguinal lymph node biopsy specimen before BTK inhibitor treatment. The structure of lymph node was destroyed and replaced by infiltration of small lymphocytes, amyloid material (black arrow) was present among tumor cells (HEX200) (A). The inserted lower right picture was the magnification of tumor cells and amyloid material (HEX400). Immunohistochemical staining demonstrated the tumor cells were positive for CD20 (B), CD38 (C), IgM (D) and was negative for P53 (E) and showed a low proliferation rate by Ki-67 staining (F) (Envision X200).
FIGURE 3
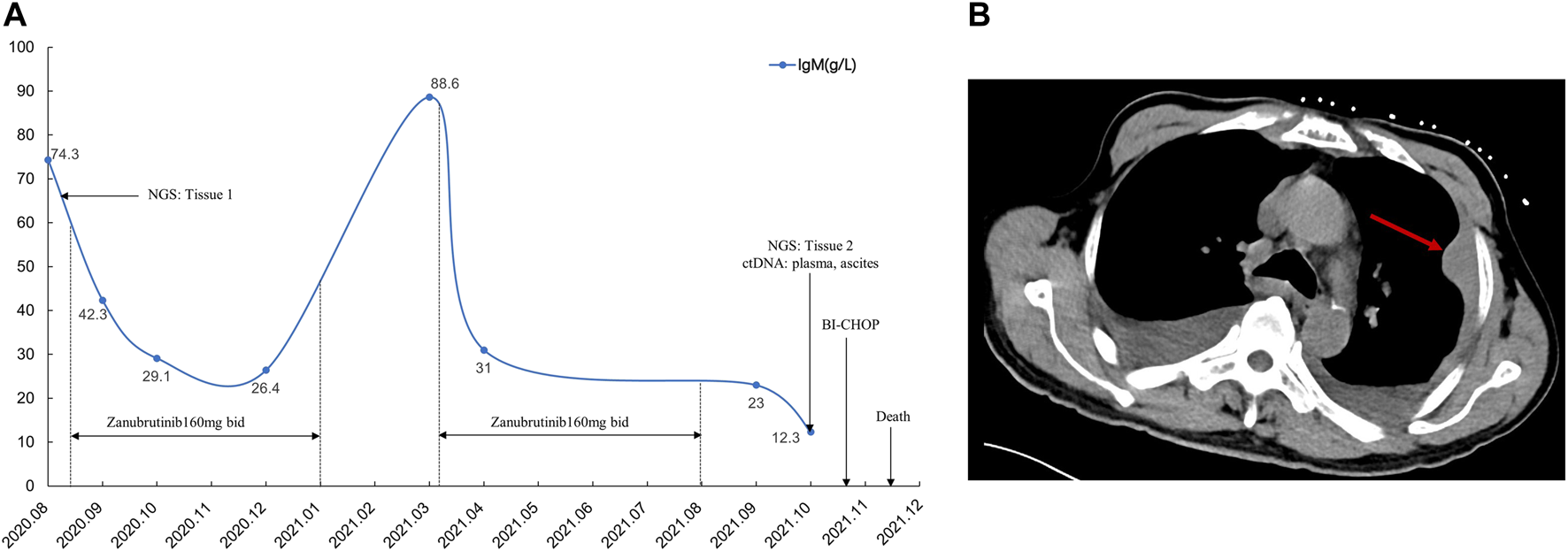
Treatment after disease recurrence and the time and type of obtained specimens over the disease course (A). The red arrow shows chest wall mass on chest CT scan (B).
Two months after zanubrutinib self-withdrawal, the patient was hospitalized with fever and pain in the left lower limb in March 2021. Laboratory examinations revealed hemoglobin at 105 g/L, platelets at 115 × 109/L, serum β-2 microglobulin at 8.32 mg/L and IgM at 88.6 g/L. HBV DNA quantification <1.0e+2 IU/mL. Imaging examination confirmed enlargement of the spleen and retroperitoneal lymph nodes. Considering that the patient’s IgM increased significantly after drug withdrawal and disease progression, we restarted 160 mg zanubrutinib twice daily. However, at 1.5 months after zanubrutinib self-withdrawal, he developed worsening abdominal distention and was readmitted in September 2021. Laboratory examinations revealed hemoglobin at 133 g/L, platelets at 93*109/L, IgM at 23.0 g/L, serum β-2 microglobulin at 5.11 mg/L; HBV DNA quantification <5.99e+6 IU/mL, and serum-ascites albumin gradient (SAAG) at 19.7 g/L. Gastroscopy and imaging examination confirmed chronic liver cirrhosis. Notably, chest CT showed a new mass in the left chest wall (8.5 × 3.1 cm) (Figure 3). Chest wall tissue biopsy showed that the tumor cells were medium to large in size with round or oval nuclei, and small nucleoli could be seen in some tumor cells. IHC demonstrated that the abnormal lymphoid cells were CD19+, CD20+, CD10−, Bcl-6+, MUM+, Bcl-2+, CD38−, CD138−, CD5−, and CD23−. The tumor cells were diffusely and strongly positive for P53. A high Ki-67 index (>90%) indicated aggressive lymphoma. Fluorescence in situ hybridization test showed that the P53 gene was lost (Figure 4). According to Hans’ criteria, these findings suggest that the patient had a non-GCB subtype of DLBCL. The international prognostic index was 4, indicating high risk. From the clinical perspective, we considered that the WM had transformed to DBCL. However, clonal homology validation was not performed because of insufficient samples and disease progression. We analyzed tumor DNA obtained from different anatomic sites (left inguinal lymph node, chest wall tissue) and ctDNA derived from plasma and ascites supernatant, which showed significant temporal and spatial heterogeneity. High-throughput sequencing analysis was performed using the whole exome region of 193 cancer-related genes (ChosenMed, Beijing, China) with MGISEQ-2000, with a mean sequencing depth of 500× and 10000× in tissue and ctDNA, respectively (Supplementary Table S1). Data were analyzed using an in-house bioinformatics pipeline. According to the NGS results, MYD88L265P was detected in left inguinal lymph node; MYD88L265P, TP53Y220D and ECT2LR255*were detected in the chest wall tissue. In addition to these mutations, we detected genetic mutations (BTKT316A, PLCG2D334G,R337W) associated with resistance to BTK inhibitors in ctDNA derived from ascites (Figure 5). However, these mutations were not detected in plasma ctDNA. Genetic testing revealed high concordance between ascites and tumor tissue, suggesting the clinical utility of ascites for liquid biopsy in WM patients. Considering his performance status and financial situation, the patient received bendamustine, ibrutinib 140 mg twice daily (reduced due to liver damage), attenuated CHOP (cyclophosphamide, pharmorubicin, vinorelbine, and dexamethasone) and antiviral therapy (entecavir). However, he did not respond to chemotherapy, and there was no significant improvement. Finally, he succumbed to progressive disease and poor performance status in November 2021. The overall survival time was 98 months.
FIGURE 4
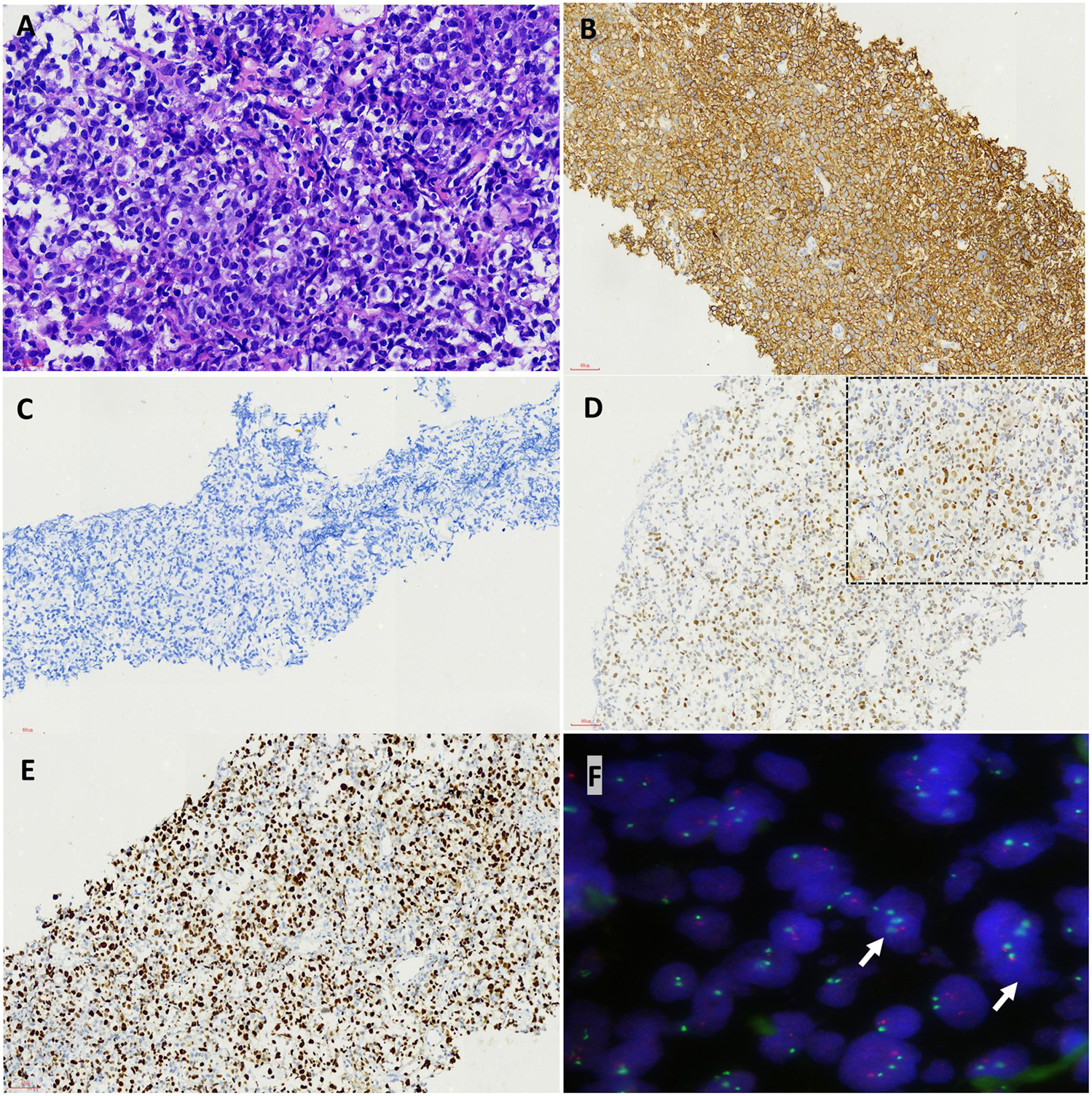
Pathological features of high grade transfomation in the biopsy from the chest wall after BTK inhibitor treatment. The tumor cells were medium to large in size with round or oval nuclei and small nucleoli could be seen in some tumor cells (HEX400) (A). Immunohistochemical staining demonstrated the tumor cells was positive for CD20 (B) but negative for CD38 (C), diffusely and strongly positive for P53 (D, inserted upper right picture was high power view, X400) and showed a much higher proliferation rate by Ki-67 staining (E) (Envision X200). FISH test showed P53 gene lost (white arrow, the red signal was P53 gene, the green signal was CEP17) (F).
FIGURE 5
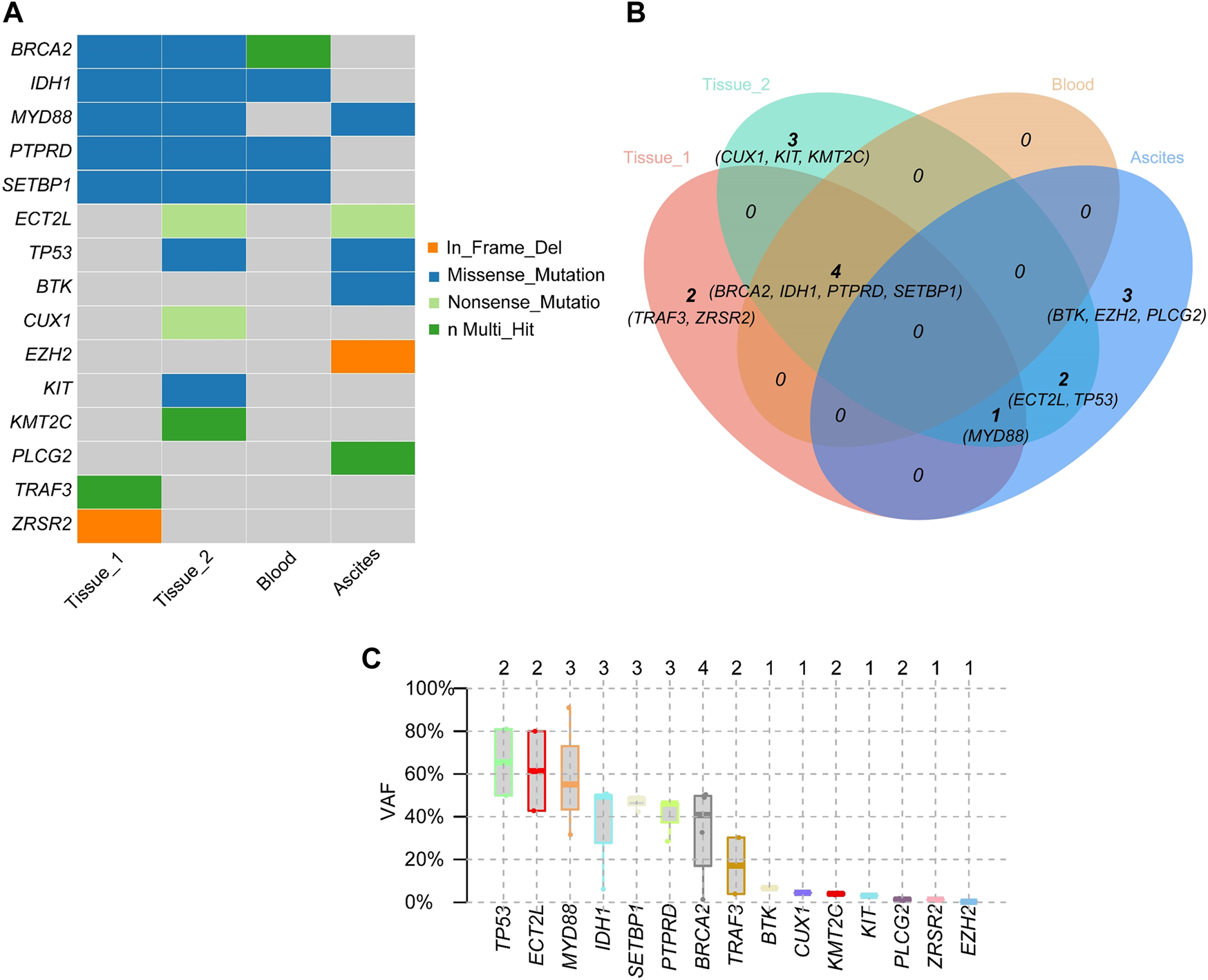
Mutated genes and gene mutation types of inguinal lymph node (Tissue 1), transformed chest wall tissue (Tissue 2), peripheral blood, and ascites (A). Relationships of mutant genes in different specimens (B). Variant Allele Frequency of mutated genes (C).
Discussion
WM is a B-cell malignancy manifested by accumulation of lymphoplasmacytic cells in bone marrow and/or lymphatic tissue. Elevated serum immunoglobulin-M (IgM) tends to correlate with disease burden and can cause symptoms associated with hyperviscosity, tissue infiltration, and autoimmune phenomena (9). WM has been associated with a variety of highly recurrent somatic mutations, including in MYD88 (95%–97%), CXCR4 (30%–40%), ARID1A (17%), and CD79B (8%–15%). These identifications of somatic mutations provide important insight into diagnosis, drug options for treatment, and monitoring of disease progression in WM (10). In recent years, precision medicine has gained popularity, integrating genetic testing into clinical care to maximize patient outcomes. The use of appropriate samples for specific genetic analysis is particularly important for patients who cannot undergo invasive procedures. Compared with traditional tissue biopsy, circulating tumor DNA (ctDNA) (commonly known as liquid biopsy) is a relatively noninvasive method that can be used for diagnosis, dynamic monitoring of therapeutic effects, revealing the emergence of drug resistance and quantification of minimal residual disease (MRD) in cancer. Numerous body fluids can be used for detection of ctDNA, including blood, urine, saliva, cerebrospinal fluid, pleural fluid and bronchial washings (11). Liquid biopsy is increasingly utilized in treatment of hematologic malignancies and has made great contributions. Many studies have illustrated the potential of liquid biopsy approaches for diagnosis, therapy, assessment of MRD, early detection of relapse and prognosis in aggressive B-cell lymphomas, acute myeloid leukemia, myelodysplastic syndrome, chronic lymphocytic leukemia and multiple myeloma (12–15). This cost-efficient method can prevent the discomfort and complexity caused by repeated bone marrow biopsy in WM. Some recent studies have shown that it is feasible to assess genomic landscapes with this minimally invasive, yet sensitive tool (5, 16). Bagratuni et al. showed higher MAFs of MYD88L265P in both peripheral blood ctDNA and bone marrow tDNA in symptomatic WM, with MAF being reduced after therapy and increased in relapsed by Cast-PCR (17). In addition to peripheral blood, MYD88L265Pmutation has been detected in skin lesions, pleural effusions and CSF in WM (18–20), thought it is rarely reported in ascites. Compared to tumor tissue and plasma, the DNA detected in ascites supernatants may derive from necrotic or apoptotic tumor cells and contain abundant tumor-derived DNA, which provides more important genomic information for different tumors (8, 21, 22). In our study, the gene mutations detected in ctDNA from ascites supernatants and chest wall tumor tissue were highly identical, whereas no significant genetic mutations were detected in plasma ctDNA. These results indicate that ascites supernatants may be suitable sample types in clinical practice for genetic and molecular analyses for WM patients with ascites.
Unexpectedly, we also detected genetic mutations (BTKT316Aand PLCG2D334G, R337W) associated with BTK inhibitor resistance in ascites derived ctDNA. BTK inhibitors are new treatment options for WM patients. Ibrutinib was the first BTK inhibitor to receive approval by the FDA for treatment of WM (23). Although ibrutinib has high therapeutic activity and leads to durable remission in WM treatment, its use has been affected by off-target effects and drug resistance (24). Compared with ibrutinib, zanubrutinib is a selective, irreversible BTK inhitior, that has less toxicity and better response quality (25). With the increasing use of BTK inhibitors, more WM patients experience resistance to treatment. Acquired resistance to ibrutinib is strongly associated with recurrent mutations in BTK (especially BTKCys481) and PLCG2. BTKT316, a mutation in the SH2 nonkinase domains of BTK, has been reported in CLL patients who experienced progression on ibrutinib. This mutation prevents critical contact with phosphotyrosine, thereby reducing the affinity of BTK to BLNK or other BTK partner proteins and leading to drug resistance. Sharma et al. identified that cells carrying BTKT316A exhibit resistance to ibrutinib at the cellular and molecular levels similar to those carrying BTKC481S (26). Studies in CLL and WM have shown that gain-of-function mutations in PLCG2(R665W, L845F, S707) are associated with ibrutinib resistance (27–29). Chen et al. confirmed that the BTKC481S mutation confers resistance to ibrutinib by activating ERK1/2 continuously and releasing inflammatory and prosurvival cytokines in MYD88-mutated WM; (30). Recently, PLCG2D334G was detected in the TIM domain of ibrutinib-resistant R/R CLL (31) and may be linked to Richter’s transformation (32). However, PLCG2(D334G, R337W) mutations have not been reported in WM. In our case, the reason why the patient developed drug resistance and histological transformation may be related to treatment-driven clonal evolution. D. Talaulikar et al. suggested that IGHV analysis is required to determine whether DLBCL tissue is clonally associated with the previous WM clone. It is important to determine whether the two tumors share the same clonal origin, as patients with clonal homologous histological transformations tend to have worse prognosis (33). Unfortunately, IGHV analysis of the patient was limited by the paucity of samples. Whether chest wall tumors have clonal homology or tend to be of independent clonal origin remains speculative. We detected the mutation of TP53 in chest wall tumor and its VAF was 80.98%. TP53 mutation predicts unfavorable prognosis despite the low frequency in WM. Poulain et al. observed that 7% of WM patients had TP53 mutation at diagnosis and more genomic abnormalities. Of note, the overall survival of WM with TP53 alterations was significantly short, especially in those with symptomatic WM, which was independent of the IPSSWM score (34). Disease progression, complications and development to high-grade lymphoma are the main causes of death in WM (35). Histological transformation of WM to DLBCL may occur rarely. Lin et al. reported that histological transformation to DLBCL occurs in 13% of WM patients, which is related to poor clinical prognosis (36). A large retrospective study of 1,466 WM patients described that WM has an approximately 2% cumulative occurrence rate of histological transformation after 10 years, with a median survival of 2.7 years (37). Durot et al. suggested that the presence of elevated LDH, constitutional symptoms and extranodal involvement, especially MYD88-associated sites, should be highly suspected of histological transformation in WM patients (38). Moreover, Jiménez et al revealed that disease transformation is largely determined by the frequency and specificity of acquired alterations. Some acquired recurrent mutations (such as in PIM1, FRYL and HNF1B) may be involved in clonal selection for disease transformation. There is potential to predict transformation risk with CD79B in WM (39). In our case, transformed DLBCL was highly aggressive and was associated with adverse prognostic factors, a high IPI score, extranodal involvement and a high Ki-67 index at diagnosis. The patient had extremely poor prognosis and died within 1.5 months after histological transformation. The course of dynamic change in IgM and comparison of pathological characteristics by both indolent and aggressive processes, and the presence of multiple genetic mutation sites supports disease progression and histological transformation to DLBCL.
Conclusion
In conclusion, ctDNA derived from ascites provides more information for genetic profiling and drug resistance than plasma or tissue. In the absence of tumor tissues, ascites may be a good alternative for genetic analysis in clinical practice in WM patients with ascites. We believe that ctDNA analysis should be performed in prospective studies to assess genomic profiling of disease processes in WM. Although clonal homology between DLBCL and antecedent WM is not clear, analysis of genetic mutations and aggressive histologic transformation features strongly suggests a clonal relationship between the two B-cell lymphomas. Since the prognosis of transformed DLBCL is worse than that of de novo DLBCL, it is important to evaluate the clonal relationship between antecedent WM and transformed DLBCL.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Tongji Hospital affiliated to Tongji University. The patient provided his written informed consent to participate in this study. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BX, YZ, and AL conceived and designed the experiment. JZ and XZ carried out the studies, participated in collecting data, and drafted the manuscript. JZ, XZ, FX, BX, and YZ participated in acquisition, analysis, or interpretation of data. YiD, HL, YaD, PL, and JF revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was sponsored by Program of Outstanding Young Scientists of Tongji Hospital of Tongji University (No. HBRC1802), Research Project of Shanghai Putuo District Eastern integrated healthcare system (YLT2103) and Clinical Research Plan of SHDC (No. SHDC2020CR6005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2023.1611070/full#supplementary-material
References
1.
Owen RG Treon SP Al-Katib A Fonseca R Greipp PR McMaster ML et al Clinicopathological definition of waldenstrom's macroglobulinemia: Consensus panel recommendations from the second international Workshop on waldenstrom's macroglobulinemia. Semin Oncol (2003) 30(2):110–5. 10.1053/sonc.2003.50082
2.
Gertz MA . Waldenström macroglobulinemia: 2021 update on diagnosis, risk stratification, and management. Am J Hematol (2021) 96(2):258–69. 10.1002/ajh.26082
3.
Treon SP Cao Y Xu L Yang G Liu X Hunter ZR . Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood (2014) 123(18):2791–6. 10.1182/blood-2014-01-550905
4.
Castillo JJ Advani RH Branagan AR Buske C Dimopoulos MA D'Sa S et al Consensus treatment recommendations from the tenth international Workshop for Waldenström macroglobulinaemia. Lancet Haematol (2020) 7(11):e827–e837. 10.1016/s2352-3026(20)30224-6
5.
Bagratuni T Ntanasis-Stathopoulos I Gavriatopoulou M Mavrianou-Koutsoukou N Liacos C Patseas D et al Detection of MYD88 and CXCR4 mutations in cell-free DNA of patients with IgM monoclonal gammopathies. Leukemia (2018) 32(12):2617–25. 10.1038/s41375-018-0197-7
6.
Jang H Choi CM Lee SH Lee S Jeong MK . Read count patterns and detection of cancerous copy number alterations in plasma cell-free DNA whole exome sequencing data for advanced non-small cell lung cancer. Int J Mol Sci (2022) 23(21):12932. 10.3390/ijms232112932
7.
Li M Chen J Zhang B Yu J Wang N Li D et al Dynamic monitoring of cerebrospinal fluid circulating tumor DNA to identify unique genetic profiles of brain metastatic tumors and better predict intracranial tumor responses in non-small cell lung cancer patients with brain metastases: A prospective cohort study (GASTO 1028). BMC Med (2022) 20(1):398. 10.1186/s12916-022-02595-8
8.
Husain H Nykin D Bui N Quan D Gomez G Woodward B et al Cell-Free DNA from ascites and pleural effusions: Molecular insights into genomic aberrations and disease biology. Mol Cancer Ther (2017) 16(5):948–55. 10.1158/1535-7163.Mct-16-0436
9.
Treon SP . How I treat Waldenström macroglobulinemia. Blood (2009) 114(12):2375–85. 10.1182/blood-2009-05-174359
10.
Treon SP Xu L Guerrera ML Jimenez C Hunter ZR Liu X et al Genomic landscape of Waldenström macroglobulinemia and its impact on treatment strategies. J Clin Oncol (2020) 38(11):1198–208. 10.1200/jco.19.02314
11.
Siravegna G Marsoni S Siena S Bardelli A . Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol (2017) 14(9):531–48. 10.1038/nrclinonc.2017.14
12.
Kwok M Wu SP Mo C Summers T Roschewski M . Circulating tumor DNA to monitor therapy for aggressive B-cell lymphomas. Curr Treat Options Oncol (2016) 17(9):47. 10.1007/s11864-016-0425-1
13.
Nakamura S Yokoyama K Shimizu E Yusa N Kondoh K Ogawa M et al Prognostic impact of circulating tumor DNA status post-allogeneic hematopoietic stem cell transplantation in AML and MDS. Blood (2019) 133(25):2682–95. 10.1182/blood-2018-10-880690
14.
Yeh P Hunter T Sinha D Ftouni S Wallach E Jiang D et al Circulating tumour DNA reflects treatment response and clonal evolution in chronic lymphocytic leukaemia. Nat Commun (2017) 8:14756. 10.1038/ncomms14756
15.
Allegra A Cancemi G Mirabile G Tonacci A Musolino C Gangemi S . Circulating tumour cells, cell free DNA and tumour-educated platelets as reliable prognostic and management biomarkers for the liquid biopsy in multiple myeloma. Cancers (Basel) (2022) 14(17):4136. 10.3390/cancers14174136
16.
Ntanasis-Stathopoulos I Bagratuni T Gavriatopoulou M Patseas D Liacos C Kanellias N et al Cell-free DNA analysis for the detection of MYD88 and CXCR4 mutations in IgM monoclonal gammopathies; an update with clinicopathological correlations. Am J Hematol (2020) 95(6):E148–E150. 10.1002/ajh.25802
17.
Bagratuni T Markou A Patseas D Mavrianou-Koutsoukou N Aktypi F Liacos CI et al Determination of MYD88L265P mutation fraction in IgM monoclonal gammopathies. Blood Adv (2022) 6(1):189–99. 10.1182/bloodadvances.2021005354
18.
Alegría-Landa V Prieto-Torres L Santonja C Córdoba R Manso R Requena L et al MYD88 L265P mutation in cutaneous involvement by Waldenström macroglobulinemia. J Cutan Pathol (2017) 44(7):625–31. 10.1111/cup.12944
19.
Gustine JN Meid K Hunter ZR Xu L Treon SP Castillo JJ . MYD88 mutations can be used to identify malignant pleural effusions in Waldenström macroglobulinaemia. Br J Haematol (2018) 180(4):578–81. 10.1111/bjh.14386
20.
Hiemcke-Jiwa LS Minnema MC Radersma-van Loon JH Jiwa NM de Boer M Leguit RJ et al The use of droplet digital pcr in liquid biopsies: A highly sensitive technique for MYD88 p.(L265P) detection in cerebrospinal fluid. Hematological Oncol (2018) 36(2):429–35. 10.1002/hon.2489
21.
Wu H Ji H Yang W Zhang M Guo Y Li B et al Liquid biopsy using ascitic fluid and pleural effusion supernatants for genomic profiling in gastrointestinal and lung cancers. BMC Cancer (2022) 22(1):1020. 10.1186/s12885-022-09922-5
22.
Zhou S Xu B Qi L Zhu D Liu B Wei J . Next-generation sequencing reveals mutational accordance between cell-free DNA from plasma, malignant pleural effusion and ascites and directs targeted therapy in a gastric cancer patient. Cancer Biol Ther (2019) 20(1):15–20. 10.1080/15384047.2018.1504720
23.
Treon SP Tripsas CK Meid K Warren D Varma G Green R et al Ibrutinib in previously treated Waldenström's macroglobulinemia. New Engl J Med (2015) 372(15):1430–40. 10.1056/NEJMoa1501548
24.
Argyropoulos KV Palomba ML . First-Generation and second-generation Bruton tyrosine kinase inhibitors in Waldenström macroglobulinemia. Hematol Oncol Clin North Am (2018) 32(5):853–64. 10.1016/j.hoc.2018.05.012
25.
Tam CS Opat S D'Sa S Jurczak W Lee HP Cull G et al A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: The ASPEN study. Blood (2020) 136(18):2038–50. 10.1182/blood.2020006844
26.
Sharma S Galanina N Guo A Lee J Kadri S Van Slambrouck C et al Identification of a structurally novel BTK mutation that drives ibrutinib resistance in CLL. Oncotarget (2016) 7(42):68833–41. 10.18632/oncotarget.11932
27.
Woyach JA Furman RR Liu TM Ozer HG Zapatka M Ruppert AS et al Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. New Engl J Med (2014) 370(24):2286–94. 10.1056/NEJMoa1400029
28.
Liu TM Woyach JA Zhong Y Lozanski A Lozanski G Dong S et al Hypermorphic mutation of phospholipase C, γ2 acquired in ibrutinib-resistant CLL confers BTK independency upon B-cell receptor activation. Blood (2015) 126(1):61–8. 10.1182/blood-2015-02-626846
29.
Xu L Tsakmaklis N Yang G Chen JG Liu X Demos M et al Acquired mutations associated with ibrutinib resistance in Waldenström macroglobulinemia. Blood (2017) 129(18):2519–25. 10.1182/blood-2017-01-761726
30.
Chen JG Liu X Munshi M Xu L Tsakmaklis N Demos MG et al BTK(Cys481Ser) drives ibrutinib resistance via ERK1/2 and protects BTK(wild-type) MYD88-mutated cells by a paracrine mechanism. Blood (2018) 131(18):2047–59. 10.1182/blood-2017-10-811752
31.
Bödör C Kotmayer L László T Takács F Barna G Kiss R et al Screening and monitoring of the BTK(C481S) mutation in a real-world cohort of patients with relapsed/refractory chronic lymphocytic leukaemia during ibrutinib therapy. Br J Haematol (2021) 194(2):355–64. 10.1111/bjh.17502
32.
Maddocks KJ Ruppert AS Lozanski G Heerema NA Zhao W Abruzzo L et al Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol (2015) 1(1):80–7. 10.1001/jamaoncol.2014.218
33.
Talaulikar D Biscoe A Lim JH Gibson J Arthur C Mackinlay N et al Genetic analysis of Diffuse Large B-cell Lymphoma occurring in cases with antecedent Waldenström Macroglobulinaemia reveals different patterns of clonal evolution. Br J Haematol (2019) 185(4):767–70. 10.1111/bjh.15610
34.
Poulain S Roumier C Bertrand E Renneville A Caillault-Venet A Doye E et al TP53 mutation and its prognostic significance in waldenstrom's macroglobulinemia. Clin Cancer Res (2017) 23(20):6325–35. 10.1158/1078-0432.Ccr-17-0007
35.
Gavriatopoulou M Terpos E Kastritis E Dimopoulos MA . Current treatment options and investigational drugs for Waldenstrom's Macroglobulinemia. Expert Opin Investig Drugs (2017) 26(2):197–205. 10.1080/13543784.2017.1275561
36.
Lin P Mansoor A Bueso-Ramos C Hao S Lai R Medeiros LJ . Diffuse large B-cell lymphoma occurring in patients with lymphoplasmacytic lymphoma/Waldenström macroglobulinemia. Clinicopathologic features of 12 cases. Am J Clin Pathol (2003) 120(2):246–53. 10.1309/r01v-xg46-mfcd-vnhl
37.
Castillo JJ Gustine J Meid K Dubeau T Hunter ZR Treon SP . Histological transformation to diffuse large B-cell lymphoma in patients with Waldenström macroglobulinemia. Am J Hematol (2016) 91(10):1032–5. 10.1002/ajh.24477
38.
Durot E Tomowiak C Michallet AS Dupuis J Hivert B Leprêtre S et al Transformed Waldenström macroglobulinaemia: Clinical presentation and outcome. A multi-institutional retrospective study of 77 cases from the French innovative leukemia organization (FILO). Br J Haematol (2017) 179(3):439–48. 10.1111/bjh.14881
39.
Jiménez C Alonso-Álvarez S Alcoceba M Ordóñez GR García-Álvarez M Prieto-Conde MI et al From Waldenström's macroglobulinemia to aggressive diffuse large B-cell lymphoma: A whole-exome analysis of abnormalities leading to transformation. Blood Cancer J (2017) 7(8):e591. 10.1038/bcj.2017.72
Summary
Keywords
resistance, Waldenström macroglobulinemia, circulating tumor DNA (ctDNA), BTK inhibitor, ascites
Citation
Zhu J, Zhu X, Xie F, Ding Y, Lu H, Dong Y, Li P, Fu J, Liang A, Zeng Y and Xiu B (2023) Case report: Circulating tumor DNA technology displays temporal and spatial heterogeneity in Waldenström macroglobulinemia during treatment with BTK inhibitors. Pathol. Oncol. Res. 29:1611070. doi: 10.3389/pore.2023.1611070
Received
19 January 2023
Accepted
06 April 2023
Published
19 April 2023
Volume
29 - 2023
Edited by
Agota Szepesi, Semmelweis University, Hungary
Updates
Copyright
© 2023 Zhu, Zhu, Xie, Ding, Lu, Dong, Li, Fu, Liang, Zeng and Xiu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Zeng, yuzeng2013@163.com; Bing Xiu, xiubing1233@tongji.edu.cn
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.