Abstract
Introduction: Olanzapine (OLZ) is one of the second-generation antipsychotics drugs (APDs) used to treat several psychiatric illnesses. Olanzapine treatment is often associated with many metabolic side effects in a dose dependent manner such as obesity, dyslipidemia and insulin resistance, induction of type II diabetes and acute pancreatitis in some patients.
Methods: Hyperbaric Oxygen therapy (HBOT) was investigated as a tool to mitigate olanzapine metabolic side effects in rats. Thirty-six female Sprague Dawley (SD) rats were divided into 4 groups; rats on olanzapine treatment either exposed to hyperbaric oxygen therapy (HBOOLZ) or left without exposure (OLZ) then non-treated rats that either exposed to hyperbaric oxygen therapy or left without exposure (control). Rats received Hyperbaric Oxygen therapy for 35 days at 2.4 atmospheres absolute (ATA) for 2.5 h daily followed by intraperitoneal injection of olanzapine at 10 mg/kg or placebo.
Results: Rats on either hyperbaric oxygen therapy or olanzapine had a significant loss in body weight. Olanzapine treatment showed a decrease in serum insulin level, triglyceride, highdensity lipoprotein (HDL) cholesterol, and lipase level but an increase in fasting blood sugar (FBS), insulin resistance index (HOMA-IR) and amylase, while rats’ exposure to hyperbaric oxygen therapy reversed these effects. The Pancreatic Langerhans islets were up-regulated in both hyperbaric oxygen therapy and olanzapine treatments but the combination (HBOOLZ) doubled these islets number.
Discussion: This study advocated that hyperbaric oxygen therapy can be an alternative approach to control or reverse many metabolic disorders (MDs) associatedwith olanzapine treatment. In addition, it seems that hyperbaric oxygen therapy positively affect the pancreatic Langerhans cells activity and architecture.
Introduction
Olanzapine (OLZ), a thienobenzodiazepine derivative, it which has binding affinity for a broad range of neurotransmitter receptors. Olanzapine is a second generation of atypical antipsychotic agents (APDs) that is effective in the treatment of many disorders such as Schizophrenia, bipolar disorders whether it is mixed or manic episodes, anxiety, chemotherapy-induced nausea, sleep disorders and treatment-resistant depression [1, 2]. However, it has a high metabolic side effects, specifically when it is prescribed for long-term treatment or administrated at high doses [3, 4]. These side effects include weight gain, insulin resistance and hyperglycemia, new-onset diabetes, and diabetic ketoacidosis [5]. Moreover, olanzapine treatment is associated with altered meal patterns as well as increase the risk of dyslipidemia, decrease in fat oxidation that is secondary or pre-dispose factor to olanzapine-induced weight gain in patients [6–9].
The mechanism of antipsychotic-induced weight gain remains unclear as there are conflicting results in the literature about inducing weight gain after APDs treatment. It has been showed in animal models that the APDs cause hyperphagia followed by hyperglycemia [10], while a study reports in rats on olanzapine showed that diabetes did occur but without any weight gain [11]. Olanzapine appears to induce an increase in central body fat deposition, insulin, and triglyceride levels, suggesting that it is possible to induce insulin resistance development in rats [12]. It is generally noticed that the OLZ effect on weight gain and triglycerides serum concentration occur in a dose dependent manner as high doses treatment with OLZ causes inability to move and reach food, which thus consequently prevents weight gain compared to injections of low concentration doses [13, 14]. This effect occurs only when the drug is delivered to the animals by injection with high dose while oral administration with any doses usually results in increase in weight gain [15, 16]. In the contrary, OLZ induces insulin resistance regardless of the dose or route of administration and leads to impaired glucose tolerance especially in young population as well as has a primary risk factor for metabolic syndrome [12, 17–20]. Therefore, discontinuation of APDs has been observed and sometimes recommended to decrease the plasma glucose level [12, 21–24]. In addition, it was also documented that metabolic changes associated with OLZ treatment causes changes in pancreatic enzymes such as amylase and lipase [25, 26]. In addition, dyslipidemia and insulin resistance could be the earliest detectable metabolic abnormalities in patients treated with OLZ, which could eventually proceed to prediabetes, pancreatic β-cell failure and changes in the architecture of pancreas [11, 18, 19].
Physicians usually prescribe glucose lowering drugs such as metformin and rosiglitazone to mitigate the metabolic side effects of olanzapine therapy. Such practice is associated with some undesirable side effects. It was reported that metformin is only partially effective to reverse olanzapine-induced hyperglycemia [27] as well as results in some psychotic side effects [28]. Rosiglitazone worsen olanzapine-induced perturbations in glucose metabolism [29], thus may lead to worsening of coronary heart disease and increased risk of heart attack [30]. As an alternative approach, patients on olanzapine therapy were recommended to perform exhaustive exercise to protect against olanzapine-induced hyperglycemia and other metabolic side-effects associated with OLZ therapy [31]. However, muscle weakness and tardive dyskinesia which occur during or after treatment with olanzapine may cause difficulty in performing exercises [14, 32, 33]. Therefore, the need for unconventional approach to reduce the metabolic side effects of olanzapine that take into consideration the nature of schizophrenic clinical demonstration and side effect associated with OLZ treatment to these patients.
Due to its safe and non-invasive nature, hyperbaric oxygen treatment (HBOT) has emerged since the 1990s as a potential alternative treatment strategy for many cases such as diabetic foot ulcers, osteomyelitis, and certain infections. Based on data obtained in many studies, giving oxygen through hyperbaric therapy helps in cases of diabetes and ultimately improve a person’s quality of life through decrease in blood glucose levels and reduce insulin resistance. Oxygen is involved in cellular respiration which provides cells with their required energy to perform functions [34], in addition to its ability to improve metabolic changes [35]. Clinical studies have previously revealed the improvement in the control of hyperglycemia in patients with diabetes undergoing HBOT as well as preserved islet β-cell mass by stimulating proliferation, inhibiting apoptosis and suppressing insulitis [36]. Therefore, HBOT improves pancreatic cell function especially β-cell [37]. It also causes increment in insulin sensitivity in pancreatic tissues through its direct upregulation effect on oxidative phosphorylation process in pancreatic β-cell mitochondria that lead to upregulation in adenosine triphosphate (ATP) production. HBOT has also been reported to prevent hyperglycemia and improve muscle oxidative capacity in rodents with Type 2 diabetes [38, 39].
This study investigated the effect of HBO therapy on metabolic side effects associated with olanzapine therapy in rat model. The olanzapine-induced pathological changes in pancreatic cells also assessed in the presence and absence of HBOT. To the best of our knowledge, this is the first study that employs HBOT to control metabolic side effects associated with OLZ treatment. This approach is to replace the increase of physical activities recommendation given to patients to control side effects associated with OLZ treatment but with less burden on these patients which are usually unwilling to perform these activities due to their nature of psychotic behavior and the decrease locomotor ability following APDs treatment.
Methods and materials
Animals
Thirty-six female Sprague Dawley (SD) rats at age between 6 and 7 weeks, average weight around 140 g ± sd. The study design was approved by the Animal Care and Use Committee of Jordan University of Science and Technology which follows the animal care and uses guidelines (ILAR) [40]. All animals had free access to water and were individually caged, maintained on 12 h light–dark cycle (lights on at 07:00 h) and at a temperature of 19–22°C and 30%–40% humidity. The animals were randomly divided into 4 major groups; experimental groups received OLZ treatment and either exposed to high oxygen under pressure in the HBO chamber (i.e., HBOOLZ) (10 rats) or left without HBOT (i.e., OLZ) (10 rats). Control groups received placebo (i.e., diluent) but were either exposed to HBOT (8 rats) or left without it (i.e., Control) (8 rats).
Olanzapine treatment
Olanzapine solution was prepared by dissolving pure/raw compound of the drug (Gift from ALHikma, Jordan) in 0.1 N HCL in distilled water. The PH was adjusted at 5.5 PH with 0.01 N NaOH and the PBS was then added to reach the required final concentration (1 mg/ml). Rats received single dose of treatment intraperitoneally at 10 mg/kg daily for 35 days [41].
Oxygen therapy (HBOT) conditions
The HBOT chamber was flushed with pure oxygen from oxygen generator and then the chamber gets pressurized at a rate of 0.3 MPa (3 ATA) per 20 min. The therapy was performed on rats for 2 hours after the oxygen concentration in the chamber reached at 90%–95% with pressure inside chamber. Rats daily received HBOT between 8:00 AM and 11:00 AM o’clock before OLZ treatment for a period of 35 days.
Animal weight and samples collection
The rats were weighed weekly before entering the HBOT. The cumulative percentage of weight gain was calculated by the difference of weekly weight gain in comparison to the starting weight of each animal divided by the starting weight. Rats at the end of the experiment were fasted for 12 h before euthanization which was done by head decapitation using guillotine. Blood samples were collected after animals’ euthanization in plane tubes. Serum was then separated by centrifugation at 10,000x g 4°C for 15 min. Serum samples were stored at −20°C until analysis. Liver and pancreas were collected from these animals and directly placed in 10% neutral buffered formaldehyde in water.
Determination of the serum lipid profile and glucose concentrations
Serum total cholesterol (CHOL), low-density lipoprotein (LDL) cholesterol and triglycerides (TG) was performed according to manufacturer recommendation (Code number: 11579, 11805, 11528, respectively, BioSystems, Spain). The HDL-cholesterol (HDL-C) was analyzed using AGAPPE kit (Code number 52013001). TG, LDL and CHOL levels were evaluated in similar procedures provided by the manufacturer (Code number: 11579, 11805, 11528, respectively, BioSystems, Spain). The absorbance (A) of samples and standard was measured at 500 nm against reagent blank using spectrophotometer (UV/VIS spectrometer T80+, United States). The cholesterol concentration in the sample was calculated using the formula provided in the kits. For HDL-C measurements the absorbance of the reaction was measured at 505 nm. Serum glucose values were obtained after sampling using an enzymatic photometric test and were done in accordance of manufacturer recommendations (Code number: CH0280, ARCOMEX, Jordan). The absorbance of samples and standard was measured at 500 nm.
Enzymology
Pancreatic enzymes (i.e., amylase, lipase) and Liver enzymes (ALT and AST) were determined according to manufacturer recommendations. Amylase, ALT and AST were analyzed using Spinreact (Code number: 41202, 1001172, 1001162, respectively, Spinreact kit, Spain) while Lipase was detected using Biochemical Enterprise kit (Code number: LIP3542, Biochemical Enterprise, Italy).
Insulin and indicators of insulin resistance or sensitivity indices
Serum insulin was determined using a commercially available ELISA kit and following manufacturer recommendations (Code number: CEA448Ra, Cloud-Clone Corp, United States). All reagents and samples were brought to room temperature (18–25°C) before used. The color changes in microtiter plates were measured by ELISA reader (Biotek, United States) at 450 nm.
The following formulas was used to calculate Homeostasis model assessment of insulin resistance, the HOMA-IR index: insulin (mU/mL) X glucose (mmol/L)/22.5; and HOMA beta-cell function of the pancreas assessment, the HOMA β-cell index: (20 X fasting serum insulin (mU/mL))/(FBS (mmol/L)–3.50).
Histological evaluation
The fixed pancreas specimens (10% formaldehyde) were dehydrated in a series of ethanol treatments, starting from the 70% storing solution, and then were cleared in xylene. The blocks were serially sectioned at 7 µm with a rotary microtome (Motorized rotary microtome, United States). The sections were stained with Hematoxylin-Eosin for general morphology assessment. The sum of Langerhans islands was obtained through counting the total island appeared in three microscopic fields under ×4 magnification.
Statistical analysis
All values are displayed as mean ± S.E.M. Statistical analysis was accessed using OpenEpi (https://www.openepi.com/Menu/OE_Menu.htm). The results were compared using a one-way analysis of variance (ANOVA), and significant differences among means were tested using Student’s t-test. Only p-values less than 0.05 were considered statistically significant.
Results
Weight gain
Olanzapine treatment showed significant weight gain decrease below the control group that didn’t receive any therapy (i.e., HBOT nor OLZ) (Figure 1). The weight gain increment in control rats was 42.5% after 5 weeks of the beginning of the experiment. None of the other experimental groups that received OLZ treatment or the control rats that were placed on HBOT reached this percentage of weight gain. It was very interesting to mention that the effect of high level of oxygen exposure inside HBO chamber further hampered the growth percentage curve (weight gain) of the treated rats to almost half of the weight gain obtained in control animals by the end of the experiment. Interestingly, OLZ treated groups (i.e., with or without HBOT) caused a significant decrease in weight gain started at second weeks after treatment till the end of the experiment at 5 weeks.
FIGURE 1
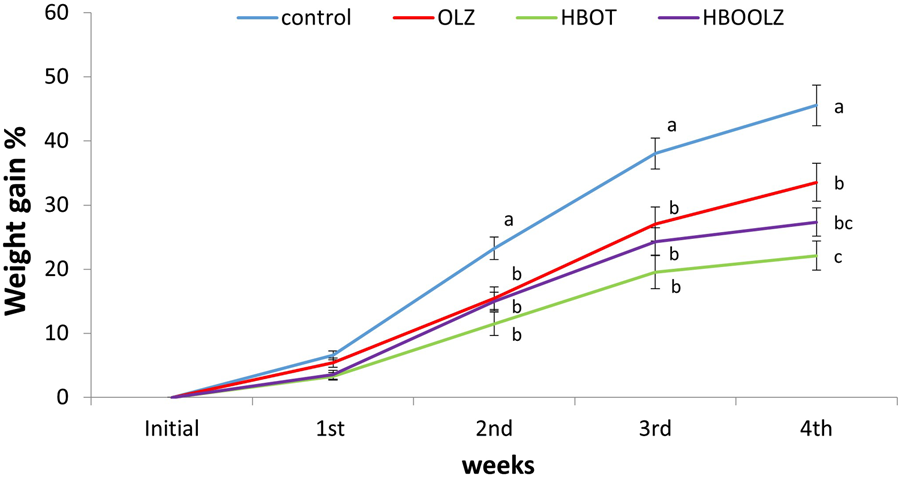
The cumulative percentage in weight gain in response to olanzapine intraperitoneal administration to SD female rats at a dose of 10 mg/kg/day in the presence and absence of HBOT. The values are means for the cumulative weight percentage ±S.E. The different letters indicate statistically significant differences between experimental groups at each time point (p < 0.05) (means shared the same letters were not significantly different).
Fasting blood sugar (FBS), insulin (INS) level and insulin resistance or sensitivity indices
The measurement of serum glucose level after fasting around 10 h (FBS) showed that there is an increment in animals that were injected by olanzapine compared with control group. The most interesting observation is that HBOT significantly lowered the glucose level in the OLZ treated group to the normal level. As well, HBOT to rats without OLZ treatment was the lowest among all experimental and control groups. On the other hand, insulin concentration in serum showed a minute difference among the groups (0.1 pg/ml). However, it was noticed that HBOT for rats caused a significant increase in insulin concentration regardless of the OLZ treatment, while OLZ treatment alone showed the lowest insulin concentration among all experimental groups (Figure 2). In regard to insulin resistance marker, rats on OLZ alone clearly had 20% higher HOMA-IR than control rats but when OLZ treatment is combined with HBOT (HBOOLZ) the HOMA-IR index return to normal levels of the control. Although serum insulin levels were comparable in all groups with slight significant decrease in OLZ group, it seems that the treatment effect get clearer when using HOMA-B index for comparison. This index was the lowest in the OLZ treated group and when HBOT is applied along with OLZ treatment the Index return to normal values indicated in control group.
FIGURE 2
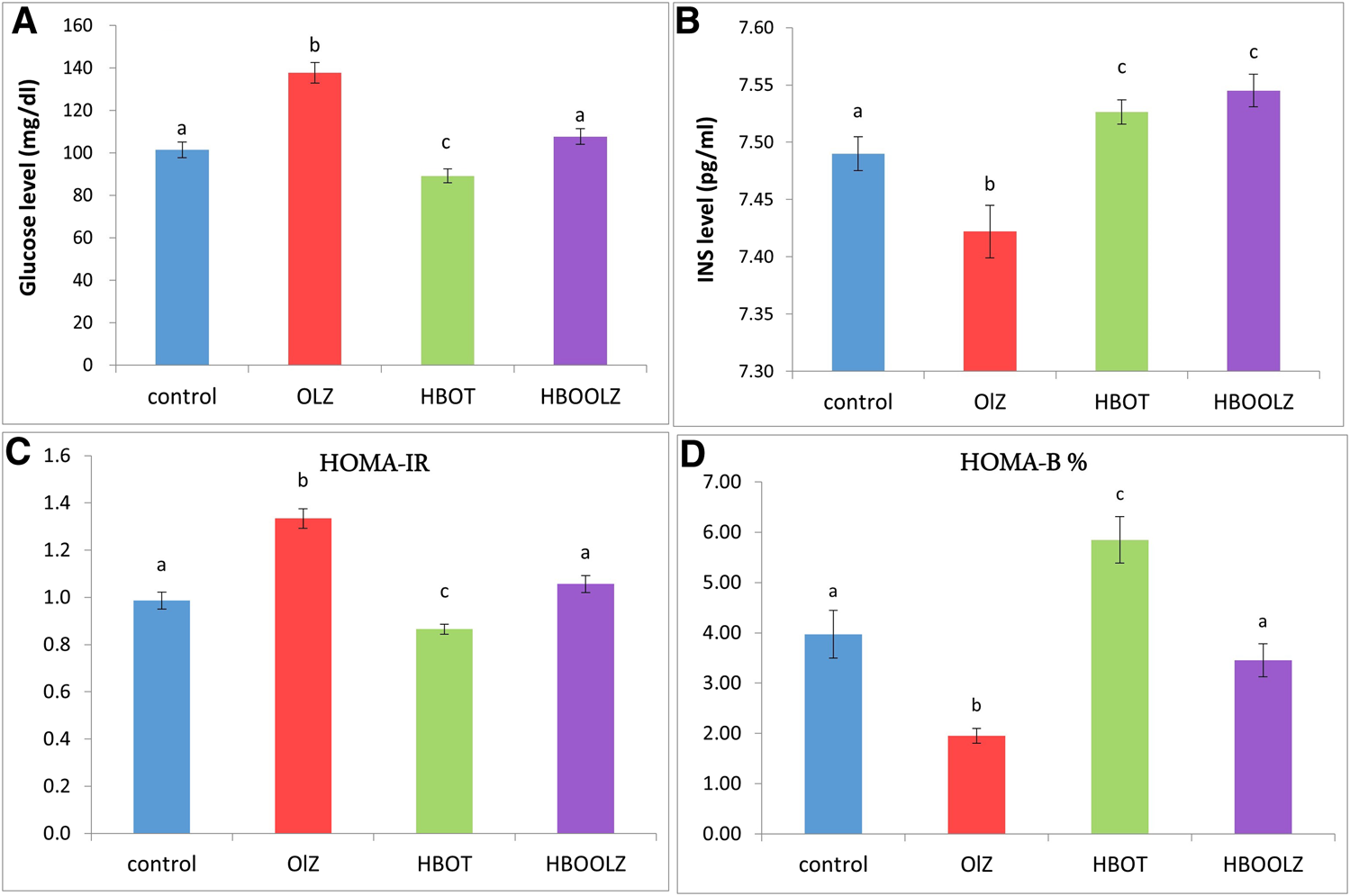
Effect of a daily single intraperitoneal dose of olanzapine (10 mg/Kg) for 5 weeks on serum fasting blood sugar (FBS) (A), insulin (INS) level (B). Insulin resistance markers in rats (HOMA-IR) (C) and insulin sensitivity markers (HOMA-B%) (D) for rats placed at different oxygen concentrations inside HBO. The values are means ± S.E. Different letters represent statistically significant differences (p < 0.05) (means shared the same letters were not significantly different).
Lipid profile such as cholesterol, triglyceride (TG), LDL and HDL
The effect of oxygen therapy and injection of olanzapine on triglyceride (TG) concentration in rat serum showed a significant down-regulation of TG in these groups compared with control group. It is important to note that this decrease was most prominent when animals were received OLZ and regardless of HBOT (Figure 3A). The combination of OLZ treatment and HBOT causes slight decrease in serum cholesterol and LDL levels in comparison to other groups (Figures 3B, D). HDL concertation positively affected by HBOT alone while OLZ treatment negatively affected its concentration regardless of the HBOT (Figure 3C). It is also very interesting to mention that the effect of OLZ injection along with HBOT (HBOOLZ) caused significant decrease in LDL when only compared to control group (Figure 3D).
FIGURE 3
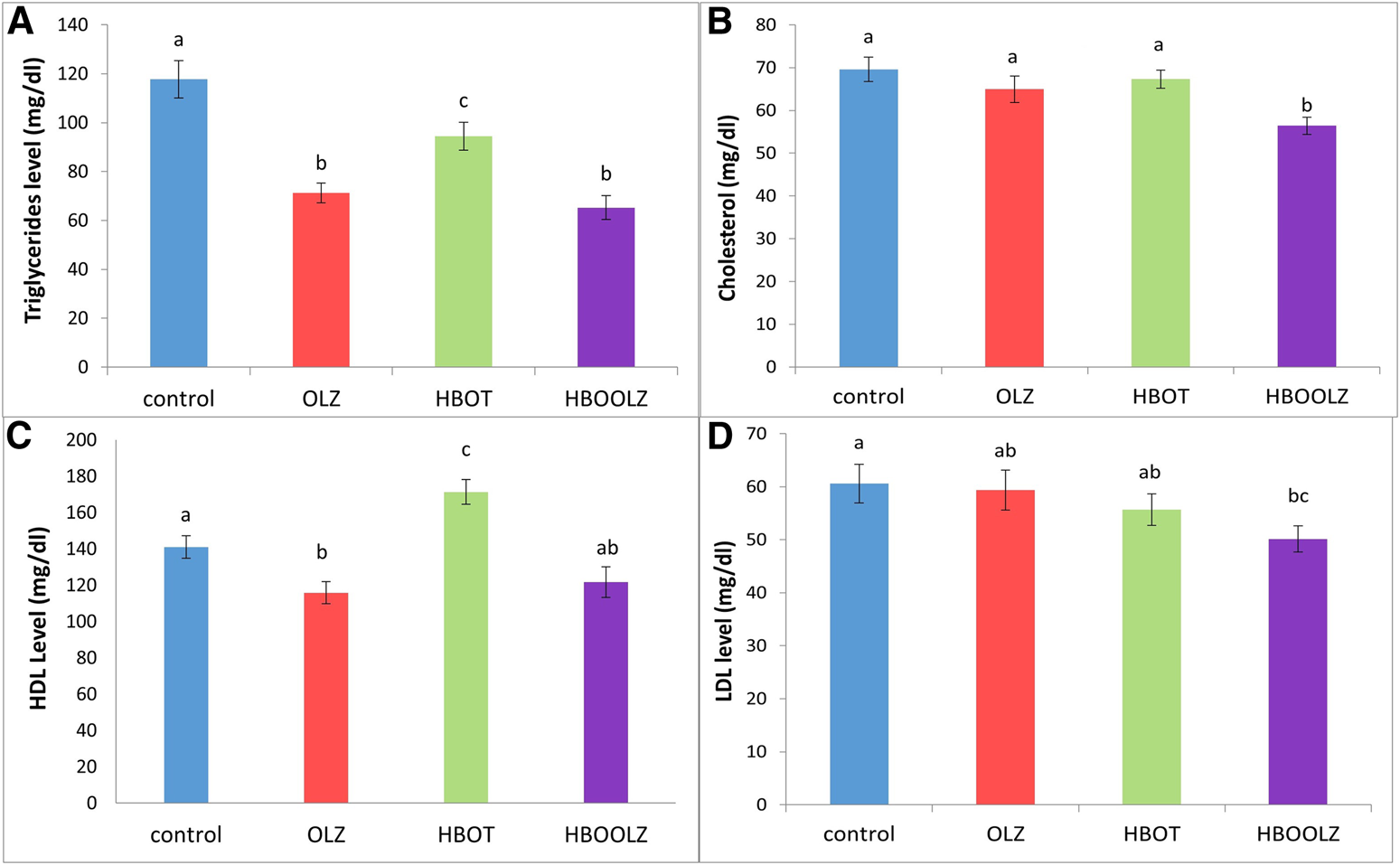
The effects of daily olanzapine intraperitoneal injection for 5 weeks on serum triglyceride (TG) (A), cholesterol (B), high density lipoprotein (HDL) (C) and low-density lipoprotein (LDL) (D) in the presence of different oxygen concentrations. The values are means ± S.E. Different letters represent statistical significant differences with a p-value less than 0.05.
Enzymatic tests
Olanzapine treatment caused significant decrease in serum lipase level, while exposure of these rats to high level of oxygen in HBO chamber can reverse this negative effect of OLZ (Figure 4A). All rats received OLZ treatment and/or HBOT showed a significant rise in serum amylase when compared with control group. Rats’ exposure to HBOT before OLZ injection (HBLOOZ group) result in decrease in amylase concentration below the level detected in OLZ treated group (Figure 4B) but still higher that the level detected in the control group.
FIGURE 4
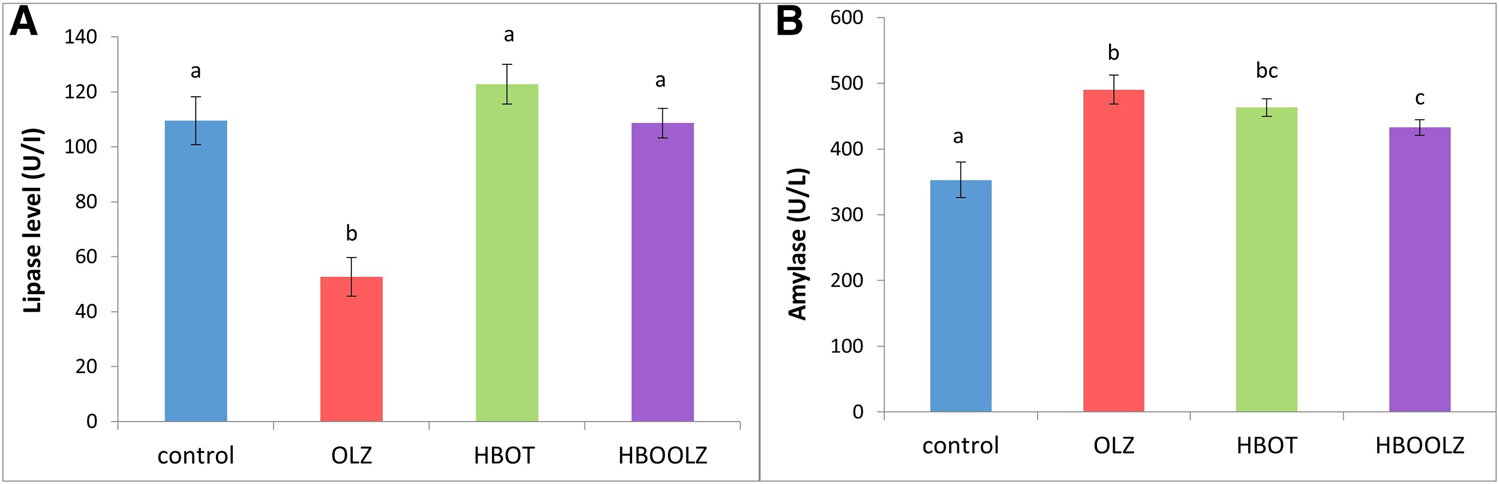
The effects of daily olanzapine intraperitoneal injection and HBOT for 5 weeks on rat’s serum pancreatic enzymes; Lipase enzyme (A) and Amylase enzyme (B). The values are means ±S.E. Different letters represent statistically significant differences (p < 0.05) (means shared the same letters were not significantly different).
Histological changes of Langerhans cells in pancreas in response of OLZ and HBOT
The effect of oxygen therapy and injection of olanzapine on number of Langerhans cells in pancreas showed a significant up-regulation in these groups compared with control group. It is important to note that the combination of OLZ treatment and HBOT almost doubled the Langerhans cells compared to normal tissue in control group (Figure 5).
FIGURE 5
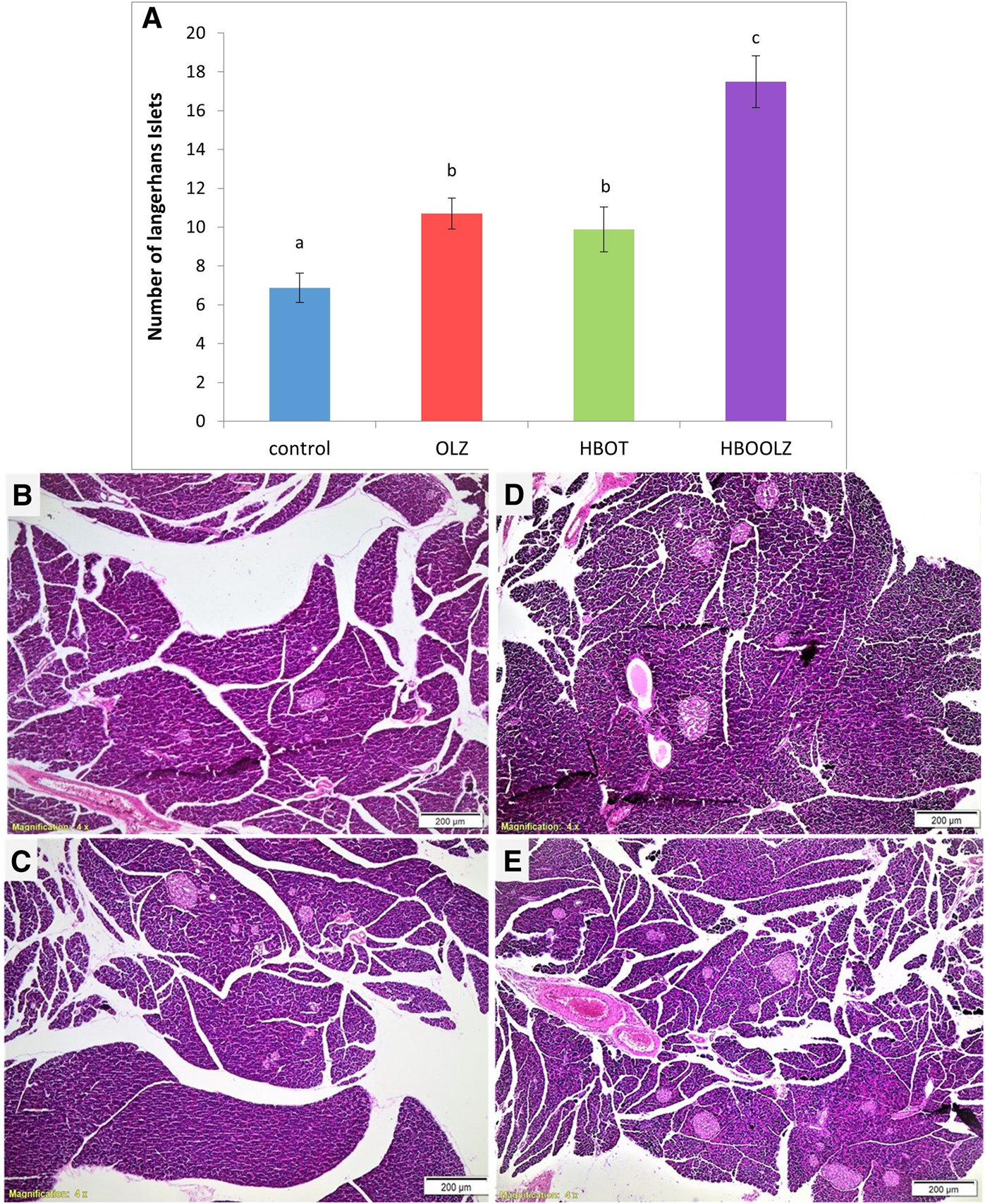
The effects of daily olanzapine intraperitoneal injection and HBOT for 5 weeks on number of Langerhans cells in pancreas (A). The values are means of the total number of islands counted in three fields at ×4 magnification for the experimental animals in each group ±S.E. Different letters represent statistically significant differences (p < 0.05) (means shared the same letters were not significantly different). Histopathological changes in Langerhans islets of pancreas due to different interventions; control rats received neither OLZ nor HBOT (B); rats injected with only OLZ (C); HBOT group only received the oxygen therapy without OLZ treatment (D); HBOOLZ received both OLZ and HBOT group (E).
Discussion
This is the first study that examined the effects of hyperbaric oxygen treatment (HBOT) on olanzapine (OLZ)-induced metabolic disorders in rat model. This work also contains some novel findings that HBOT attenuates OLZ-induced blood biological side effects. The main findings are that olanzapine treated rats actually lose weight, but continue to exhibit metabolic dysregulation which appear to be alleviated by hyperbaric oxygen treatment. The oxygen treatment also appears to result in Langerhans islet proliferation in response to different treatments’ combinations. The main side effects of OLZ that got affected by HBOT were related to metabolic disorders including lipid profile, glucose and insulin levels, weight gain as well as some enzymatic changes related to metabolism such as lipase and amylase.
OLZ treatment lead to a decreased insulin sensitivity which mainly related to β-cell dysfunction [38]. It seems that OLZ treatment upregulated the number of cells in pancreatic tissues (Langerhans islets) but made them less functional in secreting insulin as presented by low HOMA-B value. This particular negative effect not only got reversed after HBOT application but also led in tremendous increase in Langerhans islets number in HBOOLZ group. To the best of our knowledge, this is also the first study the showed a direct dysfunctional effect of OLZ on pancreatic tissues that can be alleviated by HBOT application.
It was demonstrated that subjects placed on OLZ have an increase appetite that led later to an increment in weight gain. Patients that regulate their food intake (behavioral or by other medications) appetite usually had no weight difference after OLZ therapy [39]. In animal models, OLZ effect on animals is related to gender and is dose dependent [13, 42, 43]. Thus, the weight gain after OLZ treatment is controlled through behavioral changes that can be implemented when treated subjects control their food intake or can be manipulated in animal model by changing the administrated dose, interval, and route of administration. In the current study, animals were administrated OLZ therapy that adopted a high dose of OLZ (10 mg/Kg, daily for 35 days). This high dose led to decrease in rats’ activity and willingness to move toward feeders (observational). This locomotor inhibition was noticed in all treated animals (Data not shown). Similarly, it was well documented that human patients treated with OLZ lack the tendency to perform normal daily activities. The patients who suffer from schizophrenia for instance and consuming OLZ had a lower physical activity, poor fitness, muscular strength and sedentary lifestyle in addition to an altered body balance [14, 32, 33]. Despite the unwillingness for activities in human treated with OLZ, treated patients usually with increase appetite can be easily served the required meals from family or healthcare takers. This option is not available in animal models which might explain why in many animal models treated with OLZ, some animals with low activities lose weight or had no weight difference when compared to control groups [10, 11]. Consequently, as reported in current study, rats lost weight significantly starting from the third week of treatment till the end of the experiment (Figure 1).
HBOT and OLZ treated rats had same weight gain profile until week four of the experiment which later became significantly even lower than the OLZ treated group (Figure 1). It was expected that HBOT can induce some weight loss in animals placed on this therapy [44]. In fact, the use of HBOT in the current study was first used in order to prove this weight loss benefit in OLZ treated animals so it can be later adopted as a therapy in patients suffering from weight gain problem after OLZ treatment. Unfortunately, the OLZ treatment program that used high dose in this study could not mimic the same picture present in human subjects in regard to weight gain. However, the dose and the route of administration (intraperitoneal) applied in the current study considered the highest dose in rat model that can give the most side effects associated with OLZ treatments in rats [13] but it seems that it particularly did not induce weight gain in rats because of the decrease activity and subsequent decrease in food intake.
In relation to the previous findings, serum lipids were not elevated in the OLZ treated rats as expected in human subjects but instead was similar to the control group or even decreased compared specifically TG (Figure 3). This proves that the lipid profile disturbance associated with OLZ treatment is a direct effect of increase body weight due to increase in food consumption, which did not occur in this model. As mentioned in our methodology, this experiment is intended to study the changes happening while using high dose OLZ. High doses are well known to reduce movement and reduce food intake, which usually ends by internal consumption of fat [39]. HBOT effect on serum lipids was the most striking in the current study. The previous work showed that HBOT was useful in decreasing several metabolic disorders associated with increase lipid consumption in rat weight gain model [44]. Some parameters as cholesterol and LDL were still clearly decreased when OLZ treatment is associated with HBOT therapy. Therefore, the HBOT can be considered as a therapeutic approach to control hyperlipidemia problem associated with OLZ treatment in human subject. Further studies still need to proof this effect when the OLZ treatment when given at low dose to induce other metabolic disorders such as weight gain and hyperlipidemia.
The main findings in the current work are that olanzapine treated rats actually lose weight, but continue to exhibit metabolic dysregulation (fasting glucose and insulin levels) which appear to be mitigated by hyperbaric oxygen treatment. A study stated that OLZ is associated with high blood sugar levels which may be reversible after discontinuation of treatment [21, 45, 46]. It has been hypothesized that OLZ can decrease the responsiveness of the pancreatic β-cells or even induce pancreatic cell apoptosis in animal models or in vitro [11, 22, 47]. However, most human studies did not relate hyperglycemia associated with OLZ treatment with altered pancreatic β-cell histopathological changes in vivo [48]. It is generally considered that the development of diabetes after OLZ occur due to inducing obesity and subsequent insulin resistance [12, 18, 19]. However, in some patients treated with OLZ, hyperglycemic developed without reporting weight gain which is similar to certain extent to the data reported in the current study [49, 50].
It was revealed that the serum fasting blood sugar (FBS) significantly increased in the rats that treated with OLZ, while applying HBO therapy effectively alleviated such changes. Hereby, the groups of rats which were treated in the hyperbaric oxygen (HBO) chamber alone showed a significant decrease in the level of FBS slightly below the level detected in control rats, while rats which were placed in HBO chamber then injected with OLZ showed that the FBS level returned to the normal level. This outcome might be due to increase in the level of insulin in serum when exposing animals to high level of oxygen under pressure in HBO chamber. It was also noticed that HBOT to rats caused a significant increase in insulin concentration above control levels regardless of the OLZ treatment. The OLZ treatment alone showed the lowest insulin concentration among all experimental groups. It is known that a high level of insulin is accompanied by a decrease in the level of glucose in the serum. This is what was shown in a previous study in which patients were given HBOT for 2 h, six sessions per week for 5 weeks had an increase in insulin sensitivity within 3 days of hyperbaric oxygen treatment [51]. Similarly, HBOT was able to improve insulin sensitivity and reverse insulin resistance along with many other MDs parameters rats with abdominal obesity, induced by sucrose induced metabolic syndrome in rats [52].
The insulin level after OLZ treatment alone in the current work was slightly down regulated but it still within the normal physiological level in rats. This normal insulin level did not though result in controlling the significantly upregulated serum glucose level in OLZ treated group which is translated by the increased HOMA-IR index in these animals. Particularly, blockage of various receptors by olanzapine involves the regulation of glucose metabolism independent of their effects on body weight. Olanzapine blocks serotonin 5-HT2, histamine H1, α-1 adrenergic, D2 dopamine receptor (D2R) and muscarinic M3 receptors (M3R) [53]. These receptors regulates glucose uptake by muscle tissues (e.g., H1) [54], glucose tolerance (e.g., α-1 adrenergic) [55], glucose-stimulated insulin secretion by pancreatic β-cells and tissue insulin resistance and diabetes (D2R and M3R) [56–60] which clearly reflected by low HOMA-B index in OLZ treated rats in the current study. The findings reported in the current study presented insulin-resistance effect of OLZ while application of HBOT were able to alleviate these effect probably through activation of various receptors mentioned above which all need further investigations.
In the current study, the Langerhans Islet number was proliferated in pancreatic tissues after treatment with OLZ and HBOT (Figure 5). To the best of our knowledge, no in vivo study on OLZ showed such effect on Langerhans Islet. Thus, the increase in number of Langerhans Islet in pancreatic tissues of these rats may suggest that the pancreas is probably going into hyperplasia to compensate for the insulin resistance which is usually associated with OLZ treatment. The islet of Langerhans usually undergoes major structural and functional changes to control glucose level. It was reported that after HBOT therapy many adaptive changes that results in enhancement of glucose stimulated insulin secretion, β-cell proliferation, glucose oxidation and cAMP metabolism. It seems that HBOT had a significant effect on the number of islets that result in normalizing glucose concentrations and put insulin sensitivity back to normal levels. The present study provides evidence that HBOT therapy mitigated the metabolic side-effects related to OLZ treatment and specifically seems to control insulin resistance probably through proliferation of the Langerhans Islet in pancreatic tissues and increase their activities.
The type of cells that got proliferated need further analysis, as the main two types of cells present in the islets, β-cells and α-cells have opposite effects. The current data in two different treatment groups (HBOT and OLZ) gave similar effect on islets proliferation but resulted in different outcome on glucose and insulin production. Therefore, close analysis of islets of Langerhans should be examined in further studies along with the investigation of whether HBOT can reverse the reported antagonistic effect of OLZ on various receptors involve in the regulation of glucose metabolism and uptake.
In the present study, the activity of pancreatic enzymes in rats’ serum was affected by the OLZ treatment, the amylase activity increased while lipase enzyme activity decreased. The reduction of serum lipase is associated with decrease TG in animals administered OLZ. In other words, less feed intake means less demand on pancreas to release lipase. This is clearly related with the low TG presence in serum after OLZ treatment reported in this study. However, it was clearly demonstrated that up-regulation in serum amylase is related to the presence of acute Pancreatitis [61]. Therefore, the decrease in amylase production when OLZ treatment is combined with HBOT indicates that this therapy can control the acute pancreatitis associated with OLZ treatment [25, 62]. As well, our results are consistent with other studies that found HBOT had beneficial effects on inflammatory disease such as acute pancreatitis [63–65]. Therefore, close analysis for inflammatory process in the pancreas is worth later investigation.
A study recommend that people who take olanzapine, especially for long periods of time, should exercise to improve their clinical symptoms, quality of life and depressive symptoms [66] Unfortunately, most subjects could not regularly exercise due to poor motivation as the main reason or muscle weakness and tardive dyskinesia (uncontrolled body movements) which occur during or after treatment with olanzapine. On the other hand, the current results showed consistent findings with other studies that found HBOT had beneficial effects on the biochemical and histological abnormalities related to metabolic disorders and even can improve several pathological processes that are relevant to acute pancreatitis. Therefore, we can recommend adoption of HBOT before OLZ administration to improve metabolic dysregulation and others side effect that associated with OLZ treatment. As this study was novel in introducing such protocol in animal model, further studies need to replicate this research in human patients to adopt a proper protocol in human. The HBO individual chambers can be then owned by patients and the therapy can be adapted and applied at home as daily routine protocol to alleviate patients undesired side effects associated with OLZ treatment and as an alternative to indoors exercising devices.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Animal Care and Use Committee of Jordan University of Science and Technology ILAR. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This research was funded by Deanship of Research at Jordan University of Science and Technology (grant number: 21/2021).
Acknowledgments
Authors acknowledge the technical help of animal care takers at the Animal house, Jordan University of Science and Technology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Thomas K Saadabadi A . Olanzapine in: StatPearls (2018). Treasure Island, FL: StatPearls Publishing.
2.
Citrome L McEvoy JP Todtenkopf MS McDonnell D Weiden PJ . A commentary on the efficacy of olanzapine for the treatment of schizophrenia: The past, present, and future. Neuropsychiatr Dis Treat (2019) 15:2559–69. 10.2147/NDT.S209284
3.
Gautam S Meena PS . Drug-emergent metabolic syndrome in patients with schizophrenia receiving atypical (second-generation) antipsychotics. Indian J Psychiatry (2011) 53(2):128–33. 10.4103/0019-5545.82537
4.
Shirzadi AA Ghaemi SN . Side effects of atypical antipsychotics: extrapyramidal symptoms and the metabolic syndrome. Harv Rev Psychiatry (2006) 14(3):152–64. 10.1080/10673220600748486
5.
Tandon R . Safety and tolerability: how do newer generation “atypical” antipsychotics compare?Psychiatr Q (2002) 73(4):297–311. 10.1023/a:1020464017021
6.
Allison DB Mentore JL Heo M Chandler LP Cappelleri JC Infante MC et al Antipsychotic-induced weight gain: A comprehensive research synthesis. Am J Psychiatry (1999) 156(11):1686–96. 10.1176/ajp.156.11.1686
7.
Ganguli R . Weight gain associated with antipsychotic drugs. J Clin Psychiatry (1999) 60:20–4.
8.
Tadger S Melamed Y . Weight gain due to long term antipsychotic treatment of persistent mental disorders. Psychiatr Danub (2008) 20(1):37–41.
9.
Birkenaes AB Birkeland KI Engh JA Faerden A Jonsdottir H Ringen PA et al Dyslipidemia independent of body mass in antipsychotic-treated patients under real-life conditions. J Clin Psychopharmacol (2008) 28(2):132–7. 10.1097/JCP.0b013e318166c4f7
10.
Cope M Nagy TR Fernandez JR Geary N Casey DE Allison DB . Antipsychotic drug-induced weight gain: development of an animal model. Int J Obes (2005) 29(6):607–14. 10.1038/sj.ijo.0802928
11.
Shah R Subhan F Ali G Ullah I Ullah S Shahid M et al Olanzapine induced biochemical and histopathological changes after its chronic administration in rats. Saudi Pharm J (2016) 24(6):698–704. 10.1016/j.jsps.2015.06.006
12.
Haupt DW Newcomer JW . Hyperglycemia and antipsychotic medications. J Clin Psychiatry (2001) 62(27):15–26; discussion 40-1.
13.
Fernø J Ersland KM Duus IH Gonzalez-Garcia I Fossan KO Berge RK et al Olanzapine depot exposure in male rats: dose-dependent lipogenic effects without concomitant weight gain. Eur Neuropsychopharmacol (2015) 25(6):923–32. 10.1016/j.euroneuro.2015.03.002
14.
van der Zwaal EM Luijendijk MCM Evers SS la Fleur SE Adan RAH . Olanzapine affects locomotor activity and meal size in male rats. Pharmacol Biochem Behav (2010) 97(1):130–7. 10.1016/j.pbb.2010.05.009
15.
Kinon BJ Kaiser CJ Ahmed S Rotelli MD Kollack-Walker S . Association between early and rapid weight gain and change in weight over one year of olanzapine therapy in patients with schizophrenia and related disorders. J Clin Psychopharmacol (2005) 25(3):255–8. 10.1097/01.jcp.0000161501.65890.22
16.
Basson BR Kinon BJ Taylor CC Szymanski KA Gilmore JA Tollefson GD . Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. J Clin Psychiatry (2001) 62(4):231–8. 10.4088/jcp.v62n0404
17.
Zhang Z-J Yao ZJ Liu W Fang Q Reynolds GP . Effects of antipsychotics on fat deposition and changes in leptin and insulin levels: magnetic resonance imaging study of previously untreated people with schizophrenia. Br J Psychiatry (2004) 184(1):58–62. 10.1192/bjp.184.1.58
18.
Deng C . Effects of antipsychotic medications on appetite, weight, and insulin resistance. Endocrinol Metab Clin North Am (2013) 42(3):545–63. 10.1016/j.ecl.2013.05.006
19.
Martins PJ Haas M Obici S . Central nervous system delivery of the antipsychotic olanzapine induces hepatic insulin resistance. Diabetes (2010) 59(10):2418–25. 10.2337/db10-0449
20.
Ahuja N PalaNichamyN, Mackin P Lloyd A . Olanzapine-induced hyperglycaemic coma and neuroleptic malignant syndrome: case report and review of literature. J Psychopharmacol (2010) 24(1):125–30. 10.1177/0269881108096901
21.
Kohen I Gampel M Reddy L Manu P . Rapidly developing hyperglycemia during treatment with olanzapine. Ann Pharmacother (2008) 42(4):588–91. 10.1345/aph.1K607
22.
Koller EA Cross JT Doraiswamy PM Malozowski SN . Pancreatitis associated with atypical antipsychotics: from the food and drug administration's MedWatch surveillance system and published reports. Pharmacotherapy (2003) 23(9):1123–30. 10.1592/phco.23.10.1123.32759
23.
Waage C Carlsson H Nielsen EW . Olanzapine-induced pancreatitis: A case report. JOP (2004) 5(5):388–91.
24.
Wirshing DA Spellberg BJ Erhart SM Marder SR Wirshing WC . Novel antipsychotics and new onset diabetes. Biol Psychiatry (1998) 44(8):778–83. 10.1016/s0006-3223(98)00100-0
25.
Kerr TA Jonnalagadda S Prakash C Azar R . Pancreatitis following olanzapine therapy: A report of three cases. Case Rep Gastroenterol (2007) 1(1):15–20. 10.1159/000104222
26.
Ustohal L Mayerova M Valkova B Sedlakova H Kasparek T . Asymptomatic elevation of amylase and lipase after olanzapine treatment. J Clin Psychopharmacol (2016) 36(2):181–3. 10.1097/JCP.0000000000000460
27.
Remington GJ Teo C Wilson V Chintoh A Guenette M Ahsan Z et al Metformin attenuates olanzapine-induced hepatic, but not peripheral insulin resistance. J Endocrinol (2015) 227(2):71–81. 10.1530/JOE-15-0074
28.
Venkatasubramanian G Arasappa R Rao NP Behere RV Gangadhar BN . Adjuvant metformin worsens psychosis in schizophrenia: A case report. Prim Care Companion J Clin Psychiatry (2010) 12(2):PCC.09l00801. 10.4088/PCC.09l00801yel
29.
Boyda HN Procyshyn RM Tse L Hawkes E Jin CH Pang CCY et al Differential effects of 3 classes of antidiabetic drugs on olanzapine-induced glucose dysregulation and insulin resistance in female rats. J Psychiatry Neurosci (2012) 37(6):407–15. 10.1503/jpn.110140
30.
Ahmadian M Suh JM Hah N Liddle C Atkins AR Downes M et al PPARγ signaling and metabolism: The good, the bad and the future. Nat Med (2013) 19(5):557–66. 10.1038/nm.3159
31.
Castellani LN Peppler WT Miotto PM Bush N Wright DC . Exercise protects against olanzapine-induced hyperglycemia in male C57BL/6J mice. Sci Rep (2018) 8(1):772–11. 10.1038/s41598-018-19260-x
32.
Brown S Birtwistle J Roe L Thompson C . The unhealthy lifestyle of people with schizophrenia. Psychol Med (1999) 29(3):697–701. 10.1017/s0033291798008186
33.
Lieberman JA Stroup TS McEvoy JP Swartz MS Rosenheck RA Perkins DO et al Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med (2005) 353(12):1209–23. 10.1056/NEJMoa051688
34.
Semenza GL . Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J (2007) 405(1):1–9. 10.1042/BJ20070389
35.
Wilson DF Erecinska M Drown C Silver IA . The oxygen dependence of cellular energy metabolism. Arch Biochem Biophys (1979) 195(2):485–93. 10.1016/0003-9861(79)90375-8
36.
Faleo G Fotino C Bocca N Molano RD Zahr-Akrawi E Molina J et al Prevention of autoimmune diabetes and induction of β-cell proliferation in NOD mice by hyperbaric oxygen therapy. Diabetes (2012) 61(7):1769–78. 10.2337/db11-0516
37.
Zakiyah A Anggraini VS . Hyperbaric oxygen therapy to reduce blood glucose level on patients diabetes mellitus. J Nurs Care (2019) 2(1). 10.24198/jnc.v2i1.19306
38.
Grajales D Vazquez P Ruiz-Rosario M Tuduri E Mirasierra M Ferreira V et al The second-generation antipsychotic drug aripiprazole modulates the serotonergic system in pancreatic islets and induces beta cell dysfunction in female mice. Diabetologia (2022) 65(3):490–505. 10.1007/s00125-021-05630-0
39.
Huang J Hei GR Yang Y Liu CC Xiao JM Long YJ et al Increased appetite plays a key role in olanzapine-induced weight gain in first-episode schizophrenia patients. Front Pharmacol (2020) 11:739. 10.3389/fphar.2020.00739
40.
National Research Council (US). Guide for the care and use of laboratory animals. Washington, DC (1996).
41.
Mann S Chintoh A Giacca A Fletcher P Nobrega J Hahn M et al Chronic olanzapine administration in rats: effect of route of administration on weight, food intake and body composition. Pharmacol Biochem Behav (2013) 103(4):717–22. 10.1016/j.pbb.2012.12.002
42.
Davey KJ O'Mahony SM Schellekens H O'Sullivan O Bienenstock J Cotter PD et al Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology (2012) 221(1):155–69. 10.1007/s00213-011-2555-2
43.
Pouzet B Mow T Kreilgaard M VelSchow S . Chronic treatment with antipsychotics in rats as a model for antipsychotic-induced weight gain in human. Pharmacol Biochem Behav (2003) 75(1):133–40. 10.1016/s0091-3057(03)00042-x
44.
Alazaizeh RA . Improve health parameters and reduce in body weight by oxygen therapy (exercises while breathing). In: Applied biology. Jordan: Jordan University of Science and Technology (2020). p. 133.
45.
Nakamura M Nagamine T . Severe hyperglycemia induced by olanzapine was improved with a recovery of insulin secretion after switching to risperidone and introducing insulin therapy. Intern Med (2010) 49(23):2635–7. 10.2169/internalmedicine.49.4397
46.
Nakamura M Masaoka Y Nagamine T . Olanzapine-induced severe hyperglycemia was completely reversed by the restoration of insulin secretion after switching to aripiprazole and initiating insulin therapy. Clin Neuropsychopharmacol Ther (2014) 5:29–33. 10.5234/cnpt.5.29
47.
Doucette DE Grenier JS Robertson PS . Olanzapine-induced acute pancreatitis. Ann Pharmacother (2000) 34(10):1128–31. 10.1345/aph.19390
48.
Hou P-H Chang GR Chen CP Lin YL Chao IS Shen TT et al Long-term administration of olanzapine induces adiposity and increases hepatic fatty acid desaturation protein in female C57BL/6J mice. Iran J Basic Med Sci (2018) 21(5):495–501. 10.22038/IJBMS.2018.22759.5780
49.
Mir S Taylor D . Atypical antipsychotics and hyperglycaemia. Int Clin Psychopharmacol (2001) 16(2):63–73. 10.1097/00004850-200103000-00001
50.
Nagamine T . Hypoglycemia associated with insulin hypersecretion following the addition of olanzapine to conventional antipsychotics. Neuropsychiatr Dis Treat (2006) 2(4):583–5. 10.2147/nedt.2006.2.4.583
51.
Wilkinson D Chapman I Heilbronn L . Hyperbaric oxygen therapy improves peripheral insulin sensitivity in humans. Diabet Med (2012) 29(8):986–9. 10.1111/j.1464-5491.2012.03587.x
52.
Cruz-Villanueva SR Ramirez-Nava JC Moreno-Luna JA Cardenas-Urena KG Espin-Iturbe LT Sanchez Otero MG et al Effect of hyperbaric oxygen therapy (HBOT) on insulin resistance associated with abdominal obesity in wistar rats with dietary sucrose-induced metabolic syndrome. J Nutr Sci Vitaminol (2021) 67(5):292–300. 10.3177/jnsv.67.292
53.
Finkel S . Pharmacology of antipsychotics in the elderly: A focus on atypicals. J Am Geriatr Soc (2004) 52:S258–65. 10.1111/j.1532-5415.2004.52602.x
54.
Thomas J Linssen M van der Vusse GJ Hirsch B Rosen P Kammermeier H et al Acute stimulation of glucose transport by histamine in cardiac microvascular endothelial cells. Biochim Biophys Acta (1995) 1268(1):88–96. 10.1016/0167-4889(95)00049-x
55.
Shi T Papay RS Perez DM . The role of α1-adrenergic receptors in regulating metabolism: increased glucose tolerance, leptin secretion and lipid oxidation. J Recept Signal Transduct Res (2017) 37(2):124–32. 10.1080/10799893.2016.1193522
56.
Wei H Zapata RC Lopez-Valencia M Aslanoglou D Farino ZJ Benner V et al Dopamine D2 receptor signaling modulates pancreatic beta cell circadian rhythms. Psychoneuroendocrinology (2020) 113:104551. 10.1016/j.psyneuen.2019.104551
57.
Farino ZJ Morgenstern TJ Maffei A Quick M De Solis AJ Wiriyasermkul P et al New roles for dopamine D 2 and D 3 receptors in pancreatic beta cell insulin secretion. Mol Psychiatry (2020) 25(9):2070–85. 10.1038/s41380-018-0344-6
58.
Ballon JS Pajvani UB Mayer LE Freyberg Z Freyberg R Contreras I et al Pathophysiology of drug induced weight and metabolic effects: findings from an RCT in healthy volunteers treated with olanzapine, iloperidone, or placebo. J Psychopharmacol (2018) 32(5):533–40. 10.1177/0269881118754708
59.
Weston-Green K Huang X-F Deng C . Second generation antipsychotic-induced type 2 diabetes: A role for the muscarinic M3 receptor. CNS drugs (2013) 27(12):1069–80. 10.1007/s40263-013-0115-5
60.
Weston-Green K Huang XF Lian J Deng C . Effects of olanzapine on muscarinic M3 receptor binding density in the brain relates to weight gain, plasma insulin and metabolic hormone levels. Eur Neuropsychopharmacol (2012) 22(5):364–73. 10.1016/j.euroneuro.2011.09.003
61.
Qiu L Yin G Cheng L Fan Y Xiao W Yu G et al Astragaloside IV ameliorates acute pancreatitis in rats by inhibiting the activation of nuclear factor-κB. Int J Mol Med (2015) 35(3):625–36. 10.3892/ijmm.2015.2070
62.
Naxakis S Wafer M Collins R . Olanzapine-induced acute necrotising pancreatitis leading to recurrent multiple organ dysfunction syndrome. Gen Psychiatr (2022) 35(1):e100687. 10.1136/gpsych-2021-100687
63.
Cuthbertson CM Christophi C . Potential effects of hyperbaric oxygen therapy in acute pancreatitis. ANZ J Surg (2006) 76(7):625–30. 10.1111/j.1445-2197.2006.03793.x
64.
Christophi C Millar I Nikfarjam M Muralidharan V Malcontenti-Wilson C . Hyperbaric oxygen therapy for severe acute pancreatitis. J Gastroenterol Hepatol (2007) 22(11):2042–6. 10.1111/j.1440-1746.2006.03380.x
65.
Zhao H Ge B Yuan Y Wang G . Hyperbaric oxygen ameliorated acute pancreatitis in rats via the mitochondrial pathway. Dig Dis Sci (2020) 65(12):3558–69. 10.1007/s10620-020-06070-3
66.
Dauwan M Begemann MJH Heringa SM Sommer IE . Exercise improves clinical symptoms, quality of life, global functioning, and depression in schizophrenia: A systematic review and meta-analysis. Schizophr Bull (2016) 42(3):588–99. 10.1093/schbul/sbv164
Summary
Keywords
olanzapine, hyperbaric oxygen therapy, pancreatic Langerhans cells, metabolic disorders, insulin
Citation
AlQudah M, Khalifeh M, Al-Azaizeh R, Masaadeh A, Al-Rusan OM and Haddad HK (2022) Hyperbaric oxygen exposure alleviate metabolic side-effects of olanzapine treatment and is associated with Langerhans islet proliferation in rats. Pathol. Oncol. Res. 28:1610752. doi: 10.3389/pore.2022.1610752
Received
07 August 2022
Accepted
23 November 2022
Published
16 December 2022
Volume
28 - 2022
Edited by
József Tímár, Semmelweis University, Hungary
Updates
Copyright
© 2022 AlQudah, Khalifeh, Al-Azaizeh, Masaadeh, Al-Rusan and Haddad.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad AlQudah, m.alqudah12@just.edu.jo
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.