- 1Department of Internal Medicine, Loma Linda University Medical Center, Loma Linda, CA, United States
- 2Department of Pathology, Loma Linda University, Loma Linda, CA, United States
- 3Department of Oncology/Hematology, Loma Linda University, Loma Linda, CA, United States
Mantle cell lymphoma (MCL) is a rare and aggressive non-Hodgkin’s B cell lymphoma characterized by the translocation t(11;14) (q13;32) and overexpression of CCND1. MCL is immunophenotypically identified as CD20+, CD5+, CyclinD1+, CD43+, CD10−, BCL6−, and CD23−. It is often distinguished from B cell lymphomas of germinal center cell origin by the absence of CD10 expression. Here we report the unique clinicopathologic features of a patient with CD10+ MCL with gastrointestinal involvement and review current literature identifying this unique immunophenotype.
Introduction
Mantle cell lymphoma (MCL) is a rare and aggressive form of non-Hodgkin’s lymphoma (NHL) characterized by the abnormal proliferation of mature B lymphocytes. The disease, which accounts for 3–10% of all adult-onset NHL, presents predominately in men with a median age of 68 years (1, 2). Most cases are diagnosed at an advanced Ann Arbor stage and often manifest as progressive generalized lymphadenopathy, cytopenia, splenomegaly, and symptomatic extra-nodal involvement (1, 3).
MCL is genetically characterized by the translocation t(11;14) (q13;q32), which results in the overexpression of the proto-oncogene CCND1 caused by fusion to an enhancer of the immunoglobulin heavy chain (IgH) gene (1). This translocation event leads to the constitutive overexpression of cyclin D1 and is believed to be the primary pathogenic factor driving the dysregulated proliferation of pre-germinal-center B cells in the mantle zone areas of lymphoid follicles (4, 5). Morphologically, classical or nodal MCL (cMCL) is characterized by small to medium sized lymphocytes with irregular nuclei and inconspicuous nucleoli and small amount of cytoplasm arising from naïve-like mature B cells which express the oncogenic transcription factor SOX11. Comparatively, the more rare leukemic non-nodal MCL (nnMCL) is derived from SOX11-negative memory-like B cells (6, 7).
Most MCL cases are believed to originate from a CD20+ CD5+ CD43+ naïve-pre-germinal B cell population. MCL does not usually express the germinal center (GC) associated antigens such as CD10 and BCL-6 that are thus used to distinguish MCL from B cell lymphomas of germinal center origin, including follicular lymphoma, Burkitt lymphoma, and a subset of diffuse large B cell lymphoma, in addition to a subset of lymphoblastic lymphoma/leukemias (8). To date, few CD10+ MCLs have been reported in the literature (9–11). Here we describe the clinical features of a patient uniquely presenting with CD10+ cMCL and review current literature which have identified this distinctive immunophenotype.
Case Report
A 73- year-old male who underwent evaluation for normocytic anemia and microscopic hematuria was incidentally found to have multiple enlarged mesenteric lymph nodes throughout the abdomen and upper pelvis, with the largest measuring 1.2 cm in short axis. At the initial presentation, the patient denied fevers, chills, night sweats, weight loss, or abdominal pain. His clinical examination revealed no notable hepatosplenomegaly, or palpable lymphadenopathy. His lab results were significant for a hemoglobin of 10 g/dl (down from 13 one year ago), but were otherwise unremarkable. A peripheral smear at the time demonstrated no circulating blasts, or overt dysplastic features. However, a repeat CT abdomen and pelvis 10 months later demonstrated increased size and number (>50) of multiple enlarged mesenteric lymph nodes, with the largest having a confluent dimension of 3 cm.
A mesenteric lymph node biopsy was obtained, which revealed total effacement by small to medium-sized lymphocytes demonstrating an irregular nuclear border, clumped chromatin, and inconspicuous nucleoli and small amount of cytoplasm. Immunohistochemical (IHC) staining of the biopsied node demonstrated positive expression for CD5, CD20, CD79a, PAX5, BCL-2, SOX11, Cyclin-D1 and negative for CD3, CD15, CD23, CD30, and BCL-6; therefore, confirming the diagnosis of MCL, with a mean Ki-67 index 55% (40–70%). Remarkably, CD10 was found to be variably positive in about 70% of tumor cells (Figure 1). Bone marrow biopsy demonstrated scattered atypical B cells positive for CD5 and CD20 with lambda restriction, compatible with bone marrow involvement by mantle cell lymphoma. Flow cytometric analysis of the bone marrow aspirate confirmed the presence of a monotypic, lambda restricted, CD5+/CD10 dim + B cell population (Figure 2).
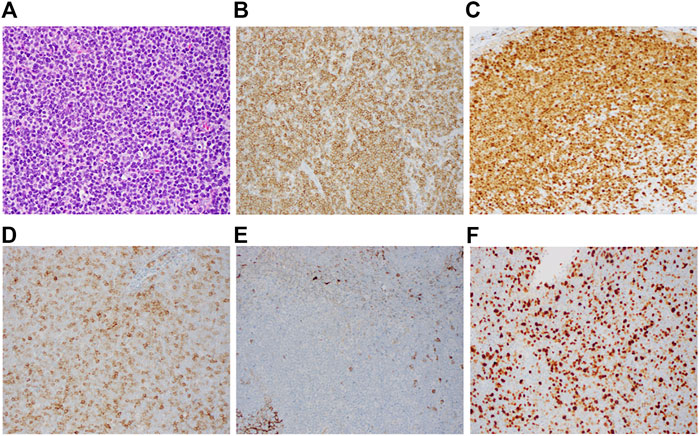
FIGURE 1. Immunohistochemistry of CD10+ mantle cell lymphoma. Representative lymph node biopsy demonstrates (A) morphologic features of the mantle cell lymphoma (H&E, x1,000) with immunostaining (B) positive for CD5, (C) positive for cyclin D1, (D) Variabily positive for CD10, (E) negative for CD23, (F) Elevated Ki-67 proliferation index (Overall 40%–70%).
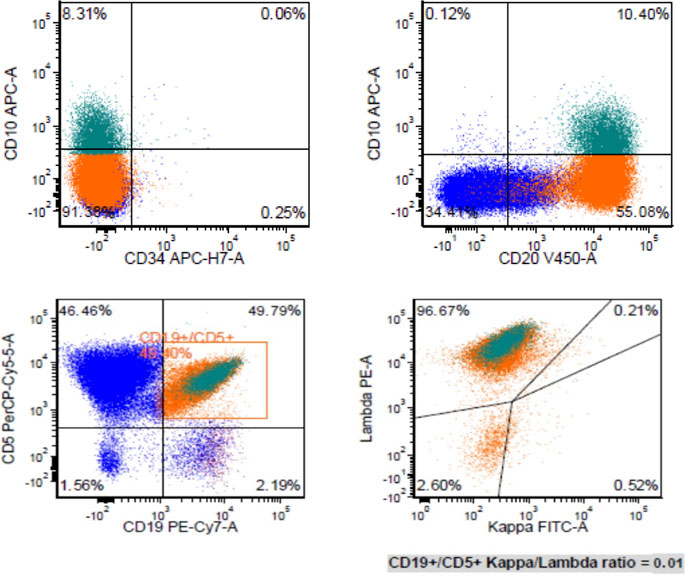
FIGURE 2. Flow cytometric analysis of bone marrow aspirate revealed a monotypic lambda restricted CD5+/CD10+ B cell population.
A positron emission tomography (PET) scan showed hypermetabolic, diffuse wall thickening of the stomach and numerous enlarged mesenteric lymph nodes/diffuse hypermetabolic activity throughout the small bowel (Figure 3). Based on the PET/CT findings, the patient underwent an esophagogastroduodenoscopy and colonoscopy. Diffuse friable, edematous, and erythematous mucosa was observed within the gastric body, greater curvature of the gastric antrum, and second part of the duodenum. Two sessile polyps were biopsied in the proximal sigmoid colon at the cecum. Immunohistochemical stains of the duodenal and proximal sigmoid colonic biopsies showed large aggregates of small to medium-sized B lymphocytes, often with irregular nuclei, expressing cyclin D1 with 40%–70% expression of the proliferative antigen, Ki-67. These findings were consistent with a diagnosis of MCL with gastrointestinal involvement. FISH analysis further confirmed the presence of t(11;14) without 17p deletion, and the absence of t(14;18) translocation. The disease was classified as stage IV with GI involvement according to the Ann Arbor staging system, MCL international prognostic index (MIPI) score was 7.6.
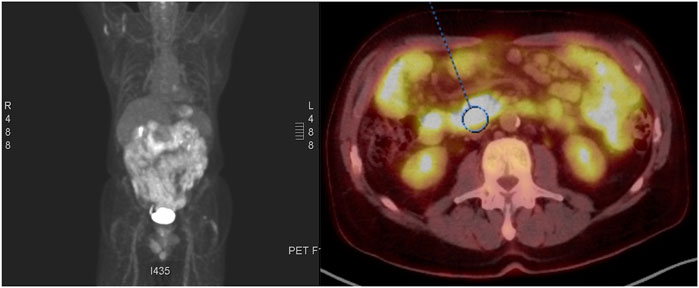
FIGURE 3. PET-CT demonstrating hypermetabolic , diffuse wall thickening of the stomach and throughout the small bowel.
The patient was initiated on induction treatment with daily administration 560 mg Ibrutinib (dose reduced to 420 mg due to hypertension) + 20 mg Venetoclax combined with escalating doses of Venetoclax to 400 mg over the first 5 weeks (12). Upon completion of 18 weeks of treatment, a repeat PET/CT showed complete remission while a bone marrow biopsy and aspiration demonstrated 30% cellularity with no evidence of lymphoma. He subsequently received autologous peripheral blood stem cell transplant (PBSCT) with successful engraftment. At follow-up a repeat colonscopy, endoscopy, and PET/CT confirmed that patient had achieved complete remssion.
Discussion
For many years, MCL was largely considered an incurable disease, with a median overall survival of 3–5 years (13). Standard therapy for patients with MCL consists of chemotherapy: R-CHOP regimen, consisting of cyclophosphamide, doxorubicin, vincristine, and prednisone combined with rituximab. However, the recent introduction of novel chemo immunotherapies in the last decade has dramatically transformed the prognostic and therapeutic landscape of MCL (14). In particular, the development of BTK inhibitors (ibrutinib, acalabrutinib, and zanubritinib), Bcl-2 antagonists (Venetoclax), and proteasome inhibitors (bortezomib) have revolutionized MCL management (15).
Recent genomic and epigenomic studies have considerably improved our understanding of the pathogenesis of MCL and demonstrated the remarkable complexity of the MCL microenvironment (7). While the t(11;14) (q13;q32) translocation leading to the overexpression of CCND1 is believed to be the primary event in the pathogenesis of the tumor, the acquisition of additional mutations in cell cycle dysregulation, DNA damage response, or cell survival pathways are important contributors to the high degree of genomic instability observed in MCL (16, 17). This in turn reflects the unique heterogeneity of clinical presentation and response to therapy. It further highlights the need to fully elucidate the clinicopathologic features that define subpopulations of MCL.
MCL classically expresses the mature B cell markers CD19, CD20, CD79a and are often distinguished from other B cell lymphomas by the expression of cyclin D1, CD5, FMC7, CD43 and the absence of CD10 and CD23 (11, 18). The case presented here describes a unique CD10 expressing MCL subpopulation that has rarely been identified in the literature. Our current understanding of CD10+ MCL is based on a limited number of case reports and studies, totaling less than 80 known cases in the literature (9–11, 19–23).
Despite the usual uniform morphology and immunophenotype, it is well recognized that cases of MCL may display clinical and molecular heterogeneity (24–29). So-called aberrant phenotypes have been described, such as CD10-positive MCL. CD10 is a transmembrane glycoprotein, normally expressed in early lymphoid progenitors and normal GC cells and widely used in lymphoma diagnosis. However, the aberrant expression of CD10 by immunohistochemistry and flow cytometry has been reported in a few cases of well-studied MCL and is believed to contribute to an even worse prognosis.
The pathologic significance of aberrant CD10 expression in MCL is highlighted by the fact that CD10 is normally expressed in early lymphoid progenitors and germinal center B cells. This raises the possibility that some MCL subpopulations may either 1) be derived from germinal center B cells, 2) be influenced by the germinal center microenvironment or 3) acquire CD10 expression through somatic mutation (24, 30). Co-expression of a second germinal center associated antigen, BCL6, has also been reported in a fraction of CD10+ MCL cases; supporting the hypothesis that at least a subset of MCL may be derived from germinal center B cells (10, 11). However, others have observed that classical MCL that transformed to blastoid MCL acquired CD10 expression at the time of transformation. In the present case, our patient was found to be CD10+ and BCL6-at the time of diagnosis with classical MCL. Previous studies have observed aberrant co-expression of Bcl-6 and CD10 in MCL (9, 10, 20). Considering that Bcl-6 expression is predominately restricted to germinal center B cells, the absence of BCL-6 in the present case may indicate that these cells did not arise from germinal center B cells (31).
Due to the paucity of reported CD10+ MCL cases, the correlation between CD10+ expression and clinical outcome has been studied to a very limited extent. A recent study by Xu et al. 2018 compared the clinicopathologic features between 30 patients with CD10+ MCL versus CD10-negative MCL patients. They had found that while CD10 expression was not independently associated with a difference in overall survival, CD10 expression was associated with an even worse prognosis in patients with a high Ki-67 (>60%), blastoid/pleomorphic morphology, or high MIPI (9). Remarkably, the patient described above with high MIPI score (7.6) and Ki-67 40%–70% was able to achieve complete response with allogeneic HSCT following Ibrutinib and ventoclax chemotherapy. The combination of the BTK inhibitor Ibrutinib and BCL2 inhibitor ventoclax has been demonstrated to have superior efficacy in patient’s primarily with relapsed/refractory MCL than those who received either Ibrutinib or ventoclax monotherapy (12). However, in the present case we show that use of combination therapy of ibrutinib and ventoclax may also be an effective therapeutic strategy in untreated cases with CD10+ phenotype. Given promising therapeutic response in the present case, future studies should further investigate how CD10+ MCL responds to this novel combination therapy.
Conclusion
Although CD10+ MCL is rare, it should be included in the differential diagnosis of CD10 + B cell lymphomas. Growing evidence suggests that CD10+ MCL is a molecularly distinct entity that requires further evaluation. Uncovering the origin of this unique subpopulation may help us to better understand the pathogenesis of MCL and spark the development of novel immunotherapies for this rare and aggressive lymphoma.
Patient Perspective
At 2 months follow-up, the patient and his family expressed satisfaction with treatment and subsequent remission.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CH, BP, and DC wrote the manuscript. DC, MA, and YL contributed to the diagnosis and treatment of case. AG and JW revised the paper. All authors read, approved the submitted version, and agreed to be accountable for all aspects of the research in ensuring the accuracy of this study. All authors have given consent to the publication of this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Jain, P, and Wang, M. Mantle Cell Lymphoma: 2019 Update on the Diagnosis, Pathogenesis, Prognostication, and Management. Am J Hematol (2019) 94(6):710–25. doi:10.1002/ajh.25487
2. Teras, LR, DeSantis, CE, Cerhan, JR, Morton, LM, Jemal, A, and Flowers, CR. US Lymphoid Malignancy Statistics by World Health Organization Subtypes: 2016 US Lymphoid Malignancy Statistics by World Health Organization Subtypes. CA Cancer J Clin (20162016) 66(6):443–59. doi:10.3322/caac.21357
3. Bosch, F, L, , pez-Guillermo, A, Campo, E, Ribera, JM, Conde, E, et al. Mantle Cell Lymphoma: Presenting Features, Response to Therapy, and Prognostic Factors. Cancer (1998) 82(3):567–75. doi:10.1002/(sici)1097-0142(19980201)82:3<567::aid-cncr20>3.0.co;2-z
5. Jares, P, Colomer, D, and Campo, E. Genetic and Molecular Pathogenesis of Mantle Cell Lymphoma: Perspectives for New Targeted Therapeutics. Nat Rev Cancer (2007) 7(10):750–62. doi:10.1038/nrc2230
6. Swerdlow, SH, Campo, E, Pileri, SA, Harris, NL, Stein, H, Siebert, R, et al. The 2016 Revision of the World Health Organization Classification of Lymphoid Neoplasms. Blood (2016) 127(20):2375–90. doi:10.1182/blood-2016-01-643569
7. Nadeu, F, Martin-Garcia, D, Clot, G, Díaz-Navarro, A, Duran-Ferrer, M, Navarro, A, et al. Genomic and Epigenomic Insights into the Origin, Pathogenesis, and Clinical Behavior of Mantle Cell Lymphoma Subtypes. Blood (2020) 136(12):1419–32. doi:10.1182/blood.2020005289
8. Ghielmini, M, and Zucca, E. How I Treat Mantle Cell Lymphoma. Blood (2009) 114(8):1469–76. doi:10.1182/blood-2009-02-179739
9. Xu, J, Medeiros, LJ, Saksena, A, Wang, M, Zhou, J, Li, J, et al. CD10-positive Mantle Cell Lymphoma: Clinicopathologic and Prognostic Study of 30 Cases. Oncotarget (2018) 9(14):11441–50. doi:10.18632/oncotarget.23571
10. Zanetto, U, Dong, H, Huang, Y, Zhang, K, Narbaitz, M, Sapia, S, et al. Mantle Cell Lymphoma with Aberrant Expression of CD10. Histopathology (2008) 53(1):20–9. doi:10.1111/j.1365-2559.2008.03060.x
11. Gao, J, Peterson, L, Nelson, B, Goolsby, C, and Chen, YH. Immunophenotypic Variations in Mantle Cell Lymphoma. Am J Clin Pathol (2009) 132(5):699–706. doi:10.1309/AJCPV8LN5ENMZOVY
12. Tam, CS, Anderson, MA, Pott, C, Agarwal, R, Handunnetti, S, Hicks, RJ, et al. Ibrutinib Plus Venetoclax for the Treatment of Mantle-Cell Lymphoma. N Engl J Med (2018) 378(13):1211–23. doi:10.1056/NEJMoa1715519
13. Herrmann, A, Hoster, E, Zwingers, T, Brittinger, G, Engelhard, M, Meusers, P, et al. Improvement of Overall Survival in Advanced Stage Mantle Cell Lymphoma. J Clin Oncol (2009) 27(4):511–8. doi:10.1200/JCO.2008.16.8435
14. Le Gouill, S, Morschhauser, F, Chiron, D, Bouabdallah, K, Cartron, G, Casasnovas, O, et al. Ibrutinib, Obinutuzumab, and Venetoclax in Relapsed and Untreated Patients with Mantle Cell Lymphoma: a Phase 1/2 Trial. Blood (2021) 137(7):877–87. doi:10.1182/blood.2020008727
15. Hanel, W, and Epperla, N. Emerging Therapies in Mantle Cell Lymphoma. J Hematol Oncol (2020) 13(1):79. doi:10.1186/s13045-020-00914-1
16. Maddocks, K. Update on Mantle Cell Lymphoma. Blood (2018) 132(16):1647–56. doi:10.1182/blood-2018-03-791392
17. Veloza, L, Ribera-Cortada, I, and Campo, E. Mantle Cell Lymphoma Pathology Update in the 2016 WHO Classification. Ann Lymphoma (2019) 3:3. doi:10.21037/aol.2019.03.01
18. Aqil, B, Triska, G, Frater, J, Hassan, A, Ruzinova, MB, Cashen, A, et al. Immunophenotypic Variations in Mantle Cell Lymphoma and Their Impact on Clinical Behavior and Outcome. Arch Pathol Lab Med (2018) 142(10):1268–74. doi:10.5858/arpa.2017-0368-OA
19. Akhter, A, Mahe, E, Street, L, Pournazari, P, Perizzolo, M, Shabani-Rad, MT, et al. CD10-positive Mantle Cell Lymphoma : Biologically Distinct Entity or an Aberrant Immunophenotype? Insight, through Gene Expression Profile in a Unique Case Series. J Clin Pathol (2015) 68(10):844–8. doi:10.1136/jclinpath-2015-202955
20. Camacho, FI, García, JF, Cigudosa, JC, Mollejo, M, Algara, P, Ruíz-Ballesteros, E, et al. Aberrant Bcl6 Protein Expression in Mantle Cell Lymphoma. Am J Surg Pathol (2004) 28(8):1051–6. doi:10.1097/01.pas.0000128671.92609.af
21. Morice, WG, Hodnefield, JM, Kurtin, PJ, and Hanson, CA. An Unusual Case of Leukemic Mantle Cell Lymphoma with a Blastoid Component Showing Loss of CD5 and Aberrant Expression of CD10. Am J Clin Pathol (2004) 122(1):122–7. doi:10.1309/UD2C-6JVP-WHXQ-Q217
22. Xu, Y, McKenna, RW, and Kroft, SH. Assessment of CD10 in the Diagnosis of Small B-Cell Lymphomas: A Multiparameter Flow Cytometric Study. Am J Clin Pathol (2002) 117(2):291–300. doi:10.1309/T88X-71U4-WC0R-2531
23. Dong, HY, Gorczyca, W, Liu, Z, Tsang, P, Wu, CD, Cohen, P, et al. B-Cell Lymphomas with Coexpression of CD5 and CD10. Am J Clin Pathol (2003) 119(2):218–30. doi:10.1309/u98advkuc26r2rja
24. Beà, S, Valdés-Mas, R, Navarro, A, Salaverria, I, Martín-Garcia, D, Jares, P, et al. Landscape of Somatic Mutations and Clonal Evolution in Mantle Cell Lymphoma. Proc Natl Acad Sci U S A (2013) 110(45):18250–5. doi:10.1073/pnas.1314608110
25. Argatoff, LH, Connors, JM, Klasa, RJ, Horsman, DE, and Gascoyne, RD. Mantle Cell Lymphoma: a Clinicopathologic Study of 80 Cases. Blood (1997) 89(6):2067–78. doi:10.1182/blood.v89.6.2067
26. Zhang, S, Jiang, VC, Han, G, Hao, D, Lian, J, Liu, Y, et al. Longitudinal Single-Cell Profiling Reveals Molecular Heterogeneity and Tumor-Immune Evolution in Refractory Mantle Cell Lymphoma. Nat Commun (2021) 12(1):2877. doi:10.1038/s41467-021-22872-z
27. Kauh, J, Baidas, SM, Ozdemirli, M, and Cheson, BD. Mantle Cell Lymphoma: Clinicopathologic Features and Treatments. Oncology (2003) 17(6):879–91.
28. Shah, BD, Martin, P, and Sotomayor, EM. Mantle Cell Lymphoma: A Clinically Heterogeneous Disease in Need of Tailored Approaches. Cancer Control (2012) 19(3):227–35. doi:10.1177/107327481201900307
29. Swerdlow, SH, and Williams, ME. From Centrocytic to Mantle Cell Lymphoma: A Clinicopathologic and Molecular Review of 3 Decades. Hum Pathol (2002) 33(1):7–20. doi:10.1053/hupa.2002.30221
30. Thelander, EF, and Rosenquist, R. Molecular Genetic Characterization Reveals New Subsets of Mantle Cell Lymphoma. Leuk Lymphoma (2008) 49(6):1042–9. doi:10.1080/10428190801947559
Keywords: case report, mantle cell lymphoma, CD10, CCND1, B-cell chronic lymphoproliferative disorders, immunophenotyping
Citation: Hino C, Pham B, Gray AL, Wang J, Castillo DR, Akhtari M and Liu Y (2022) Clinicopathologic Features and Treatment of CD10-Positive Mantle Cell Lymphoma: A Case Report and Review of Literature. Pathol. Oncol. Res. 28:1610588. doi: 10.3389/pore.2022.1610588
Received: 12 May 2022; Accepted: 28 July 2022;
Published: 25 August 2022.
Edited by:
Agota Szepesi, Semmelweis University, HungaryCopyright © 2022 Hino, Pham, Gray, Wang, Castillo, Akhtari and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Liu, eWFubGl1QGxsdS5lZHU=
 Christopher Hino
Christopher Hino Bryan Pham1
Bryan Pham1 Austin L. Gray
Austin L. Gray Dan Ran Castillo
Dan Ran Castillo