Abstract
Introduction: We aimed to explore the abnormal expression of dual-specificity protein phosphatase 1 (DUSP1) and its latent molecular mechanisms in ovarian carcinoma (OVCA).
Materials and Methods: Two clinical cohorts collected from two different hospitals were used to evaluate the expression of DUSP1 protein in OVCA tissues. RNA-sequencing and microarray datasets were utilised to verify DUSP1 expression at mRNA levels in both OVCA tissues and in the peripheral blood of OVCA patients. Furthermore, an integrated calculation was performed to pool the standard mean difference (SMD) from each cohort in order to comprehensively assess the expression of DUSP1 in OVCA. Furthermore, we examined the relationship among DUSP1, tumour microenvironment (TME), and chemotherapy resistance in OVCA. Moreover, we used pathway enrichment analysis to explore the underlying mechanisms of DUSP1 in OVCA.
Results: A pooled SMD of −1.19 (95% CI [−2.00, −0.38], p = 0.004) with 1,240 samples revealed that DUSP1 was downregulated in OVCA at both mRNA and protein levels. The area under the receiver operating characteristic curve of 0.9235 indicated the downregulated DUSP1 in peripheral blood may have a non-invasive diagnostic value in OVCA. Through six algorithms, we identified that DUSP1 may related to tumour-infiltrating T cells and cancer associated fibroblasts (CAFs) in OVCA. Pathway enrichment demonstrated that DUSP1 might participate in the mitogen-activated protein kinase (MAPK) signalling pathway. Furthermore, DUSP1 may have relations with chemotherapy resistance, and a favourable combining affinity was observed in the paclitaxel-DUSP1 docking model.
Conclusion: DUSP1 was downregulated in OVCA, and this decreasing trend may affect the infiltration of CAFs. Finally, DUSP1 may have a targeting relation with paclitaxel and participate in MAPK signaling pathways.
Introduction
Malignant ovarian tumours threaten female health outcomes, and such tumours are considered to be the third-most common genital system cancer worldwide [1]. Ovarian cancer (OVCA) is a common type of gynaecological cancer that is well known for its highly mortality and recurrence rates [2–5]. In recent decades, treatment of OVCA has seemingly improved, but such treatment is still challenging due to the complex clinical manifestations, biological features and molecular mechanisms [6–11]. Interval debulking surgery (IDS) is used to reduce the number of tumour cells and is typically followed by neoadjuvant chemotherapy (NACT), including carboplatin and paclitaxel [12–16]. But OVCA patients often still meet with an unfavourable prognosis due to late diagnosis [17–21]. Further study of the mechanism of OVCA will be helpful to find effective measures for early diagnosis.
Dual-specificity protein phosphatase 1 (DUSP1), a member of dual-specificity phosphatases, is a threonine-tyrosine dual-specificity phosphatase that serves as a crucial factor in the inactivation of different mitogen-activated protein kinase (MAPK) isoforms [22, 23]. DUSP1 is also an autophagy modulator that plays a key role in the ferroptosis in a context-dependent manner [24]. The function of DUSP1 in the growth, migration, and invasion of OVCA cells has widely attracted the attention of researchers. It is reported that DUSP1 is a novel therapeutic target for meningioma patients and that the inhibition of DUSP1 suppresses the tumour growth in vivo [25]. DUSP1 downregulates the expression of the mesenchymal marker Snail by inactivating JNK and ERK, which impairs the migration and invasion of prostate cancer [26]. A related study reported that DUSP1 remarkably suppresses the invasion and metastasis of hepatocellular carcinoma (HCC) cells [27]. Moreover, DUSP1 might serve as a promising target for improving the effective use of the treatment Gemcitabine in pancreatic cancer patients [28]. DUSP1 has garnered significant attention from gynaecologists because of its effective performance in the treatment of other cancers. Another study has shown that DUSP1 regulates the expression of P-glycoprotein in OVCA cells to induce paclitaxel resistance [29]. Researchers also found that the expression of DUSP1 is downregulated in ovarian cancer stem cells [30] and that DUSP1 is regarded as a prognostic seed gene in OVCA [31]. In addition, DUSP1 improves the development of endometrioid adenocarcinoma by activating the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway [32].
Although there has been some research done regarding the function of DUSP1 in OVCA, the mechanism of DUSP1 in OVCA still unknown. In the present study, we examined the expression status of DUSP1 protein within clinical OVCA cases via immunohistochemistry (IHC) staining; we then verified the expression level of DUSP1 mRNA in OVCA via high-throughput datasets. Furthermore, the downregulated trend of DUSP1 was verified via the peripheral blood of OVCA patients. The role of DUSP1 in the tumour microenvironment (TME) of OVCA was explored via estimating immune infiltration. Via the Kyoto Encyclopedia of Genes and Genomes (KEGG), Reactome Pathway Database, and gene ontology (GO) enrichment analysis, we studied the underlying molecular mechanisms of DUSP1 in OVCA. Moreover, we explored the relationship among drug susceptibility. DUSP1 and molecular docking revealed there was a favourable affinity between DUSP1 and paclitaxel. All of these steps aimed to explore the role of DUSP1 and its underlying molecular mechanisms in OVCA.
Materials and Methods
The Expression Status of Dual-Specificity Protein Phosphatase 1 Protein in Clinical Samples of Ovarian Cancer
OVCA and corresponding non-tumour ovarian tissues were collected from the First Affiliated Hospital of Guangxi Medical University (IHC cohort-1, n of OVCA = 60, n of non-tumour = 30) and the Second Affiliated Hospital of Guangxi Medical University (IHC cohort-2, n of OVCA = 15, n of non-tumour = 15), China. Our study has been approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (2020-KY-E-095) and the Ethics Committee of the Second Affiliated Hospital of Guangxi Medical University (2021-KY-0130). All patients signed the informed consent. Table 1 demonstrated the clinicopathological parameters of the patients in our study.
TABLE 1
| Clinicopathological parameters | n of cases | |
|---|---|---|
| Histological subtype | serous cystadenocarcinoma | 61 |
| mucinous cystadenocarcinoma | 13 | |
| Mixed | 1 | |
| Pathological grade | G1 | 15 |
| G2 | 21 | |
| G3 | 23 | |
| Unknown | 16 | |
| Age | <50 | 32 |
| ≥50 | 43 | |
| Clinical stage | I | 1 |
| II | 8 | |
| III | 31 | |
| IV | 6 | |
| Unknown | 15 | |
| T stage | T1 | 15 |
| T2 | 8 | |
| T3 | 37 | |
| Unknown | 15 | |
| Lymph node metastasis | N0 | 53 |
| N1 | 7 | |
| Unknown | 15 | |
| Metastasis | M0 | 54 |
| M1 | 6 | |
| Unknown | 15 | |
| Human epididymis protein 4 (HE4) | Negative | 26 |
| Positive | 34 | |
| Unknown | 15 |
Basic information of included patients with ovarian carcinoma in this study.
To determine the expression of DUSP1 at the protein level, we conducted IHC staining experiments with tissue samples, DUSP1 antibody (Biorbyt, orb216089, rabbit-anti human). All operations were conducted observing the manufacturer’s instruction. Formalin-fixed and paraffin-embedded tissue slides were soaked in xylene for deparaffinization. Anhydrous ethanol, 95% ethanol and 75% ethanol were used for rehydration. Ethylenediaminetetraacetic acid buffer (1:50, pH = 8.0) was carried out to retrieve antigen. After PBS soak, inactivation of endogenous peroxidase was accomplished via 3% H2O2 at 20°C for 10 min. The rabbit-anti human DUSP1 polyclonal antibody (dilution 1:200) was incubated at 37°C for 70 min. Universal mouse/rabbit secondary antibody was added into the tissue slides and placed in room temperature for 20 min, followed by PBS soak. Horseradish peroxidase-conjugated goat-anti rabbit IgG was added into the tissue slides and placed in 25°C for 20 min, followed by PBS soak. Diaminobenzidine was used to colorate and hematoxylin was used to counterstain. 75%, 85%, 95%, and 100% ethanol were carried out for dehydration and neutral gum was used to seal tissue slides. Simultaneously, we stained the tissue slides without the primary antibody (the rabbit-anti human DUSP1 polyclonal antibody) as negative controls (Supplementary Figure S1). We also prepared the positive controls following the manufacturer’s instruction, which reads prostate carcinoma tissues as positive controls (Supplementary Figure S2).
Two pathologists evaluated the scores of staining with microscopes independently. The staining intensity with no, light, moderate and strong staining gained 0, 1, 2, 3 point respectively. The scores of positive cells in visual field followed the criteria: 0%–5% (0 point), 6%–25% (1 point), 26%–50% (2 point), 51%–75% (3 point) and >75% (4 point). Finally, we calculated the IHC staining score via multiplying the intensity score and positive cells score [33, 34].
Screening OVCA-Associated High-Throughput Datasets
In order to assess the expression status of DUSP1 mRNA in ovarian carcinoma, we searched the Cancer Genome Atlas (TCGA), the Genotype-Tissue Expression (GTEx), and the International Cancer Genome Consortium (ICGC) databases for tertiary RNA-sequencing (RNA-seq) datasets of OVCA and normal ovaries. We also retrieved ArrayExpress, Sequence Read Archive (SRA), Gene Expression Omnibus (GEO), and Oncomine databases to collect microarrays. Datasets meeting the following criteria were excluded: 1) OVCA or non-tumour samples less than three; 2) cases not from Homo sapiens; 3) patient cases or cell lines that contained drugs, siRNA, or radiation; and 4) probes not matching official gene symbols. For the raw data from the microarrays, an affy package of R was ultilised to combine the raw data and conduct a robust multi-array average (RMA) algorithm [35]. Then we integrated databases from same platforms and removed the batch effects via sva package [36]. The data retrieval flow diagram is shown in Supplementary Figure S3. As of July 30, 2021, 16 datasets were included (Table 2).
TABLE 2
| Study | Test method/Platform | Country | Year | OVCA group | Non-tumor ovary controls |
|---|---|---|---|---|---|
| GSE26712 | GPL96 | United States | 2011 | 185 | 10 |
| GSE6008 | GPL96 | United States | 2007 | 99 | 4 |
| GSE105437 | GPL570 | South Korea | 2017 | 10 | 5 |
| GSE29450 | GPL570 | United States | 2011 | 10 | 10 |
| GSE18520 | GPL570 | United States | 2009 | 53 | 10 |
| GSE10971 | GPL570 | Canada | 2008 | 13 | 24 |
| GSE54388 | GPL570 | United States | 2017 | 16 | 6 |
| GSE14407 | GPL570 | United States | 2009 | 12 | 12 |
| GSE36668 | GPL570 | Norway | 2012 | 4 | 4 |
| GSE119054 | GPL19615 | China | 2019 | 6 | 3 |
| GSE66957 | GPL15048 | United States | 2015 | 57 | 12 |
| GSE146553 | GPL6244 | United States | 2020 | 46 | 9 |
| GSE124766 | GPL6480 | Germany | 2020 | 20 | 8 |
| GSE132289 | GPL20301 | United Kingdom | 2020 | 5 | 3 |
| GSE155310 | GPL18573 | United Kingdom | 2020 | 21 | 6 |
| TCGA_GTEx_OVCA | RNA-seq | United States | 2021 | 379 | 88 |
General characteristics of microarray and RNA-sequencing datasets on ovarian carcinoma.
OVCA, ovarian carcinoma.
Statistical Analysis of Dual-Specificity Protein Phosphatase 1 Expression in Ovarian Carcinoma Tissues
We used the Student’s t-test to compare the expression level of DUSP1 between OVCA and non-tumour tissues via GraphPad Prism 8 software (CA, United States). A two-tailed p value ≤0.05 indicated the differences were statistically significant. Receiver operating characteristic (ROC) curves were drawn to evaluate the discriminatory capacity of DUSP1 between OVCA and non-tumour samples. An area under curve (AUC) over 0.7 indicates a moderate discriminatory ability. We then performed an integrated study to comprehensively evaluate the expression status of DUSP1 in OVCA by pooling the data from the microarrays, RNA-seq, and in-house IHC. Stata v15.1 software (TX, United States) was utilised to calculate the standard mean difference (SMD) and a draw summary ROC (sROC) curve. The heterogeneity among the groups was assessed by a chi-square-based Q-test and I2 statistical analysis. I2 ≥ 50% and p value of Q-test ≤ 0.05 indicates existent heterogeneity and that a random effect model should be utilised to calculate pooled SMD; otherwise, a fixed effect model should be chosen. Egger’s test was used to assess the publication bias, and a p value ≥0.05 indicated no publication bias [14, 37, 38].
Relations Between Dual-Specificity Protein Phosphatase 1 Expression and Tumour Microenvironment Landscape in Ovarian Carcinoma
For estimating the TME composition, an Immunedeconv R package was used to evaluate immune infiltration. This package integrates six algorithms: EPIC, MCP-counter, xCell, quanTIseq, TIMER, and CIBERSORT [39]. The TME landscape was constructed using a combination of ggplot2 and a pheatmap package. Meanwhile, we conducted Pearson’s correlation analysis to explore the relations between DUSP1 expression and cancer associated fibroblasts (CAFs) infiltration in OVCA.
Pathway Analysis and Protein–Protein Interaction Network Construction
To make the underlying mechanisms of DUSP1 in OVCA clearer, we conducted pathway analysis. First, we calculated the Pearson’s correlation coefficient between the expression of DUSP1 and other genes in our included datasets, and the genes whose absolute value of correlation coefficient ≥0.4 were obtained as initial correlated expressed genes (CEGs) of DUSP1. The genes that appeared in at least three datasets were reckoned as ultimate correlated genes of DUSP1. Moreover, we calculated the SMD of every gene with a meta package of R and regarded the genes with p < 0.05 as differently expressed genes (DEGs) in OVCA. We then took the intersection genes of CEGs and DEGs to perform enrichment analysis [34].
We used the R package clusterProfiler to conduct GO term annotation and KEGG pathway enrichment analysis [40]. Furthermore, Reactome pathway enrichment was accomplished via the online tools KOBAS 3.0 [41].
Prediction of Chemotherapeutic Response for Ovarian Carcinoma Patients
To further explore the clinical significance of DUSP1, we predicted the chemotherapeutic response for each sample in the TCGA-OVCA cohort based on the Genomics of Drug Sensitivity in Cancer (GDSC) database. The prediction was implemented by a pRRophetic R package, which estimated the half-maximal inhibitory concentration (IC50) of every sample via ridge regression [42]. The Wilcoxon test was used to compare the IC50 of OVCA patients between the high- and low-DUSP1 expression groups.
Molecular Docking
To explore the targeting relationship between DUSP1 proteins and chemotherapeutic drugs, we performed molecular docking. First, we searched the crystal structure of DUSP1 proteins using the RCSB Protein Data Bank (RCSB PDB) database and downloaded a .pdb* file. Then, PyMOL software was ultilised to remove the nonspecific chains and solvent molecules. The 2D structures of the drugs were identified using the PubChem database, and we simultaneously used ChemBio3D software to minimise energy by an MM2 force-field-steepest descent algorithm; we then saved the structures as .mol2* files. Moreover, AutoDockTools 4 software was utilised to prepare the protein receptors and ligands according to the following steps: 1) add hydrogens to protein receptors; 2) compute and generate charges and save protein structures as .pdbqt* files; 3) generate the charges on the ligand atoms with Gasteiger models; and 4) adjust active torsions and save ligands as .pdbqt* files.
Next, non-flexible docking was conducted in AutoDock Vina, which established docking models via the Lamarckian genetic algorithm [43]. AutoDock Vina predicts the binding affinity (kcal/mol) of every conformation and outputs the result as a .pdbqt* file. Each conformation was assessed by simulating the docking of affinity-free energy between the protein receptors and ligands. Finally, we reckoned docking models with affinity energy ≤ -7 kcal/mol as ideal docking models and visualised the model with the lowest affinity energy via PyMOL.
Results
Expression Status of Dual-Specificity Protein Phosphatase 1 in Ovarian Carcinoma
Figures 1A,B demonstrated the hematoxylin-eosin (HE) staining of normal ovarian and ovarian carcinoma tissues. Under microscope, IHC staining illustrated that no high expressions of DUSP1 protein were found in OVCA tissues but rather in non-tumour tissues (Figures 1C,D); the difference was considered statistically significant (IHC cohort-1, p < 0.0001; IHC cohort-2, p = 0.0011; Figures 2A,B). Considering the multiple subtype of OVCA, we compared the expression of DUSP1 in different subtypes and pathological grades. However, no significant differences were found between the serous cystadenocarcinoma and mucinous cystadenocarcinoma, nor among the different grades and stages (Supplementary Figure S4).
FIGURE 1
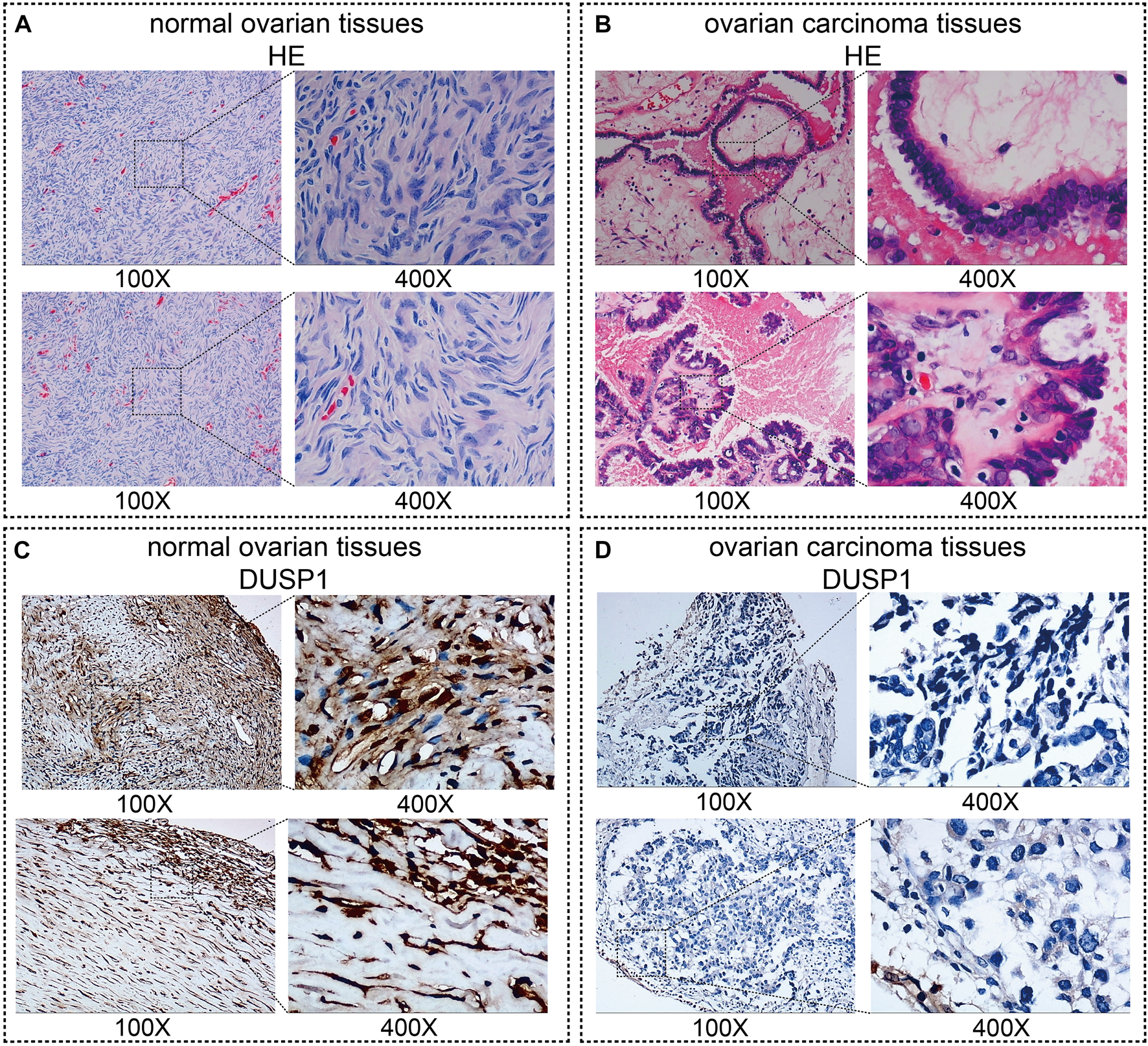
Images of normal ovarian and ovarian carcinoma tissues stained with hematoxylin-eosin (HE, (A,B)) and DUSP1 (C,D).
FIGURE 2
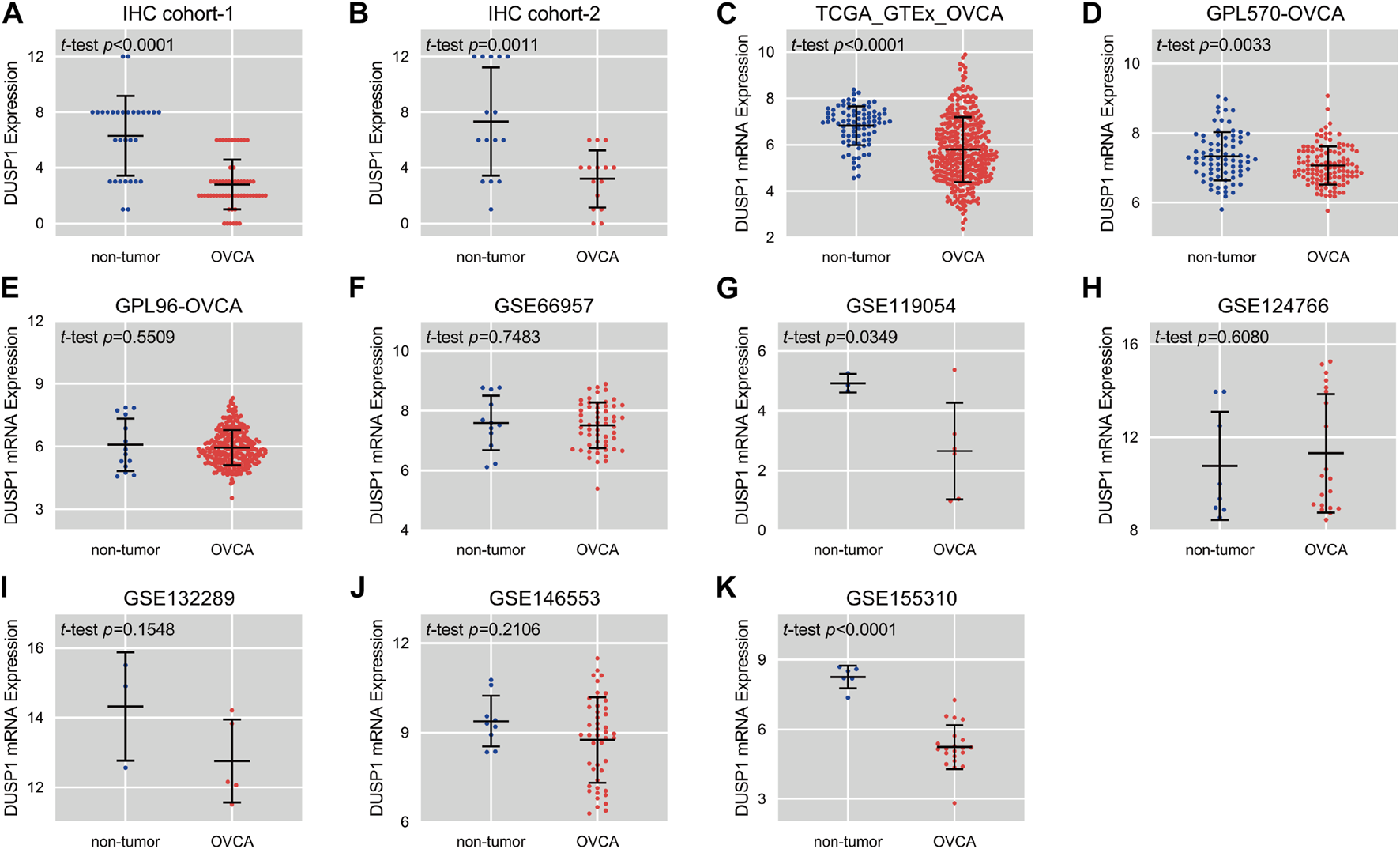
Scatter plots of the expression level of DUSP1 protein (A,B) and mRNA (C–K) in ovarian carcinoma (OVCA) and corresponding non-tumor tissues.
Expression Status of Dual-Specificity Protein Phosphatase 1 mRNA in Ovarian Carcinoma
Scatter plots showed the differences in DUSP1 mRNA expression levels between non-tumour tissues and OVCA tissues in every cohort (Figures 2C–K). Among the nine cohorts, four cohorts showed that the expression level of DUSP1 in OVCA was higher than that in non-tumour tissues, and the difference was statistically significant (TCGA_GTEx_OVCA, p < 0.0001; GPL570-OVCA, p = 0.0033; GSE11954, p = 0.0349; GSE155310, p < 0.0001). Furthermore, five other cohorts revealed no statistically significant difference in DUSP1 mRNA between OVCA and non-tumour tissues (GPL96-OVCA, p = 0.5509; GSE66957, p = 0.7483; GSE124766, p = 0.6080; GSE132289, p = 0.1548; GSE146553, p = 0.2106). The ROC curves for the above cohorts and the AUC are shown in Figures 3A–C.
FIGURE 3
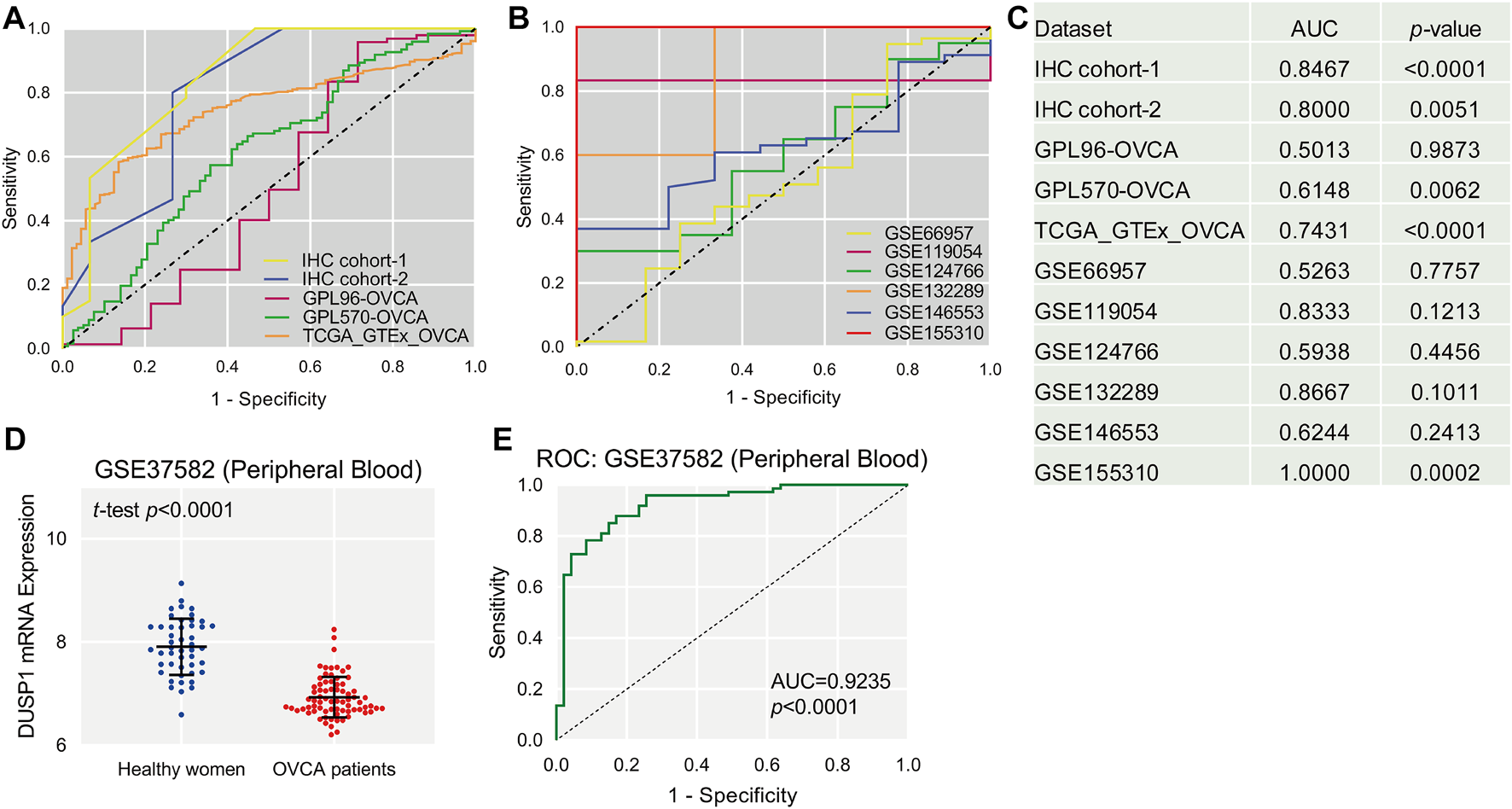
The receiver operating characteristic (ROC, (A,B)) curves of DUSP1 in ovarian carcinoma and area under curves (AUC, (C)). Scatter plot of DUSP1 mRNA expression in peripheral blood of ovarian carcinoma (OVCA) patients and healthy women (D) and ROC curve (E).
After observing the favourable discriminatory ability of DUSP1 in OVCA tissue, we evaluated the expression level and diagnostic value of DUSP1 in bodily fluids of OVCA patients. From a microarray consisting of peripheral blood lymphoblastoid cells from 74 OVCA patients and 47 healthy women, GSE37582 was obtained and was utilised to assess the expression status of DUSP1 mRNA. The expression level of DUSP1 mRNA in the peripheral blood of OVCA patients was obviously lower than that of healthy women (p < 0.0001, Figure 3D), and the area under the ROC of 0.9235 indicated a favorable diagnostic capacity of downregulated DUSP1 mRNA in peripheral blood (p < 0.0001, Figure 3E).
Comprehensive Analysis of Dual-Specificity Protein Phosphatase 1 Expression in Ovarian Carcinoma
Considering existing heterogeneity between studies (I2 = 95.6%, p < 0.0001), the random effects model was used to calculate the pooled SMD of each cohort. The result of the subgroup analysis demonstrated that DUSP1 expression level was lower in OVCA tissues than that in non-tumour tissues (mRNA subgroup: SMD = −1.14, 95% CI [−2.13, −0.14], p = 0.025; protein subgroup: SMD = -1.52, 95% CI [−1.94, −1.10], p < 0.001). The integrated SMD was −1.19 (95% CI [−2.00, −0.38], p = 0.004, Figure 4A), indicating that the expression of DUSP1 is downregulated at both mRNA and protein levels in OVCA tissues. No publication bias was found by Egger’s test (p = 0.501, Figure 4B). The AUC of the sROC curve was 0.91 (95% CI [0.89, 0.94], Figure 4C).
FIGURE 4
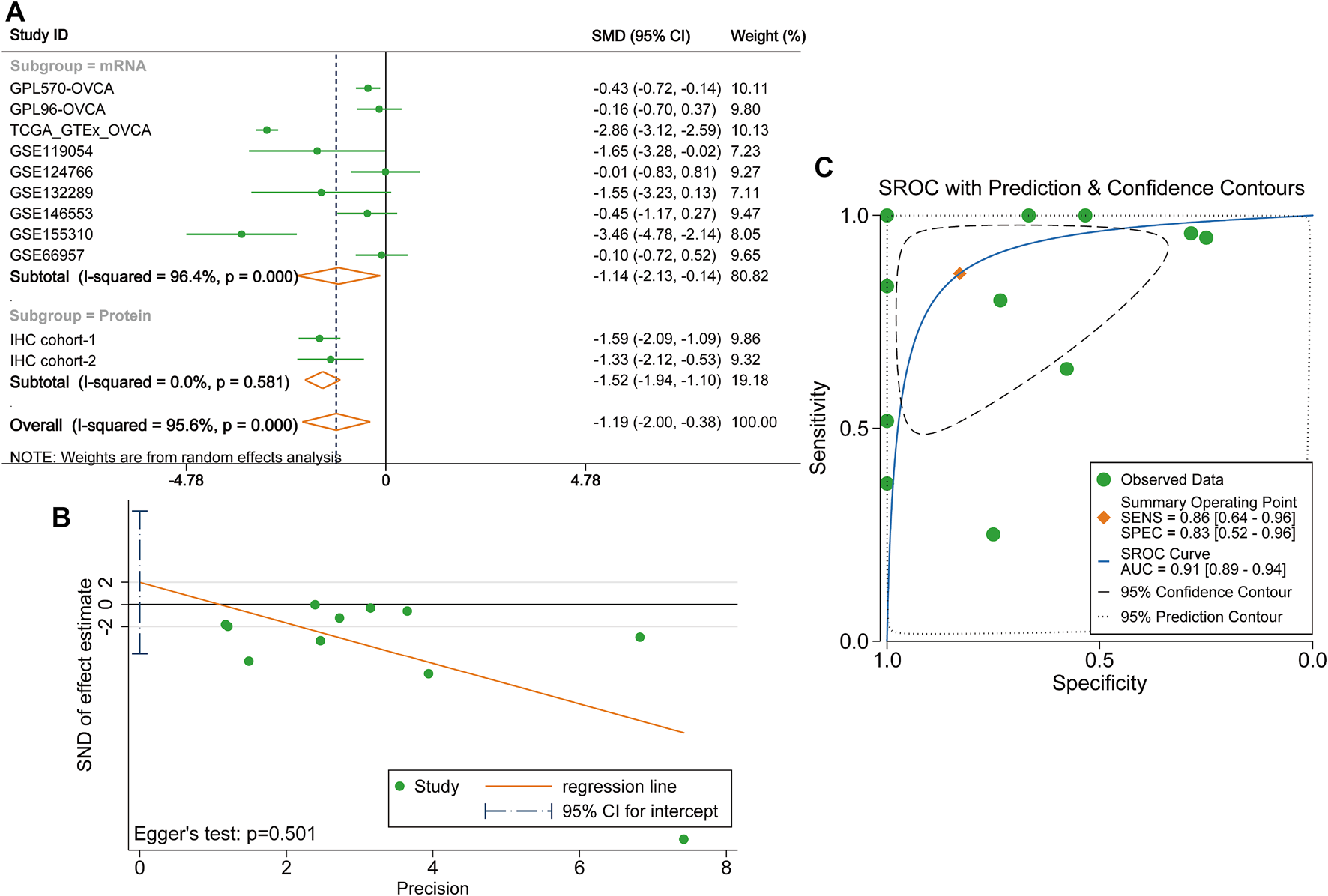
Integrated analysis on the expression of DUSP1 in ovarian carcinoma tissues. (A)Forest plot of combing standard mean difference of DUSP1 expression in ovarian carcinoma and normal ovary tissues. IHC, Immunohistochemical staining, two cohorts were from two different hospitals. OVCA, ovarian carcinoma. (B) Egger’s test for publication bias. (C) Summary receiver operating characteristic (sROC) curve of all the included cohorts.
Relations Between Dual-Specificity Protein Phosphatase 1 and Tumour Microenvironment of Ovarian Carcinoma
As illustrated in Figure 5A, six cohorts showed that DUSP1 expression was positively correlated to the infiltrating CAFs in OVCA (Pearson’s r > 0.4, p < 0.0001). Among multiple types of cells in TME, Macrophage M0 and γδT cells presented less infiltration in the high-DUSP1 groups (Figure 5B).
FIGURE 5
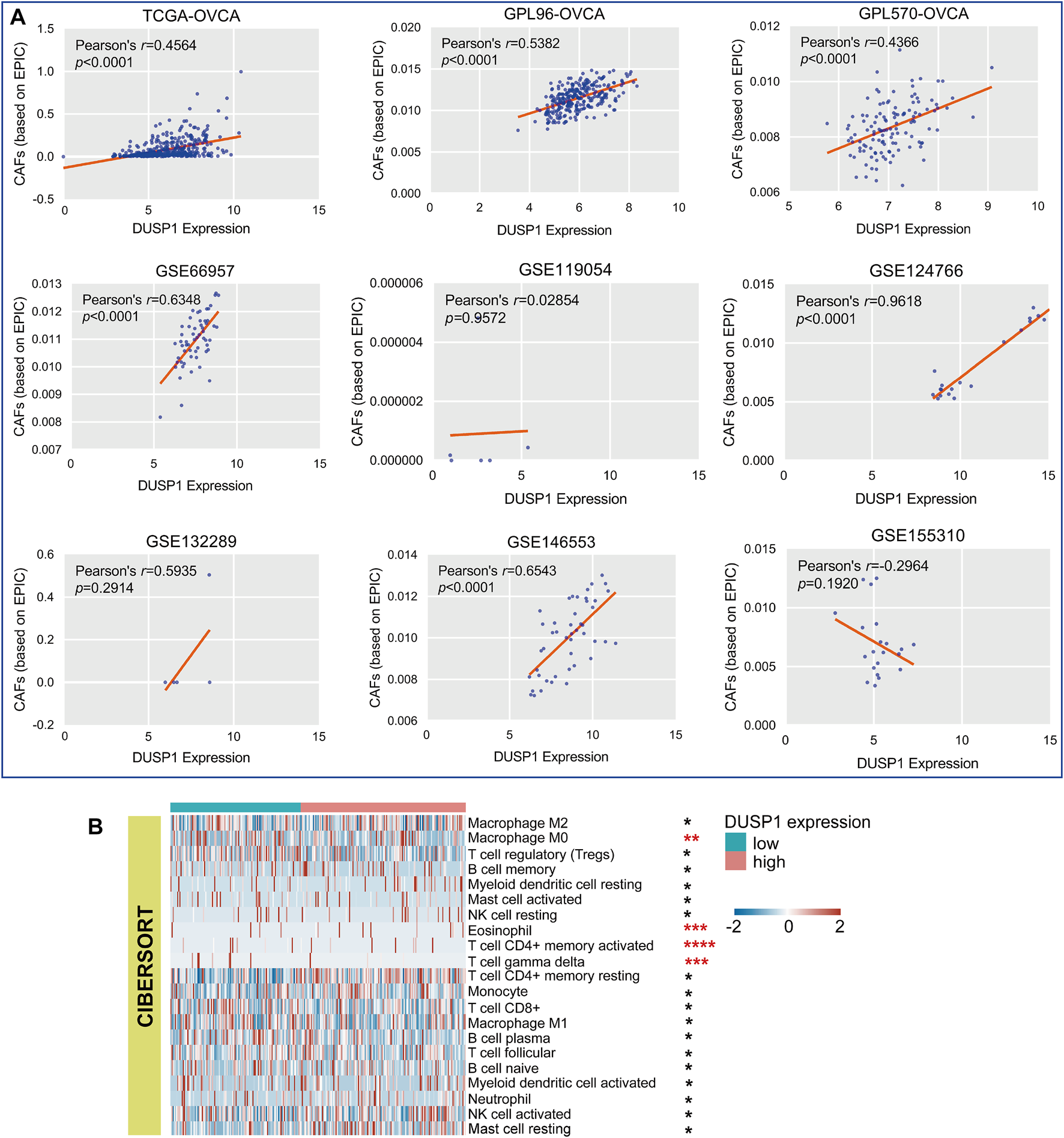
Relations of DUSP1 and tumor microenvironment in ovarian carcinoma. (A) Pearson’s correlation analysis on the DUSP1 expression and cancer associated fibroblasts (CAFs) infiltration in ovarian carcinoma tissues (based on EPIC algorithm. (B) A landscape of tumor microenvironment of ovarian carcinoma in low- and high-DUSP1 expression group based on CIBERSORT algorithms (****p ≤ 0.0001; ***p ≤ 0.001; **p < 0.05; *p ≥ 0.05).
Enrichment Analysis
After acquiring 336 downregulated positive CEGs and 64 upregulated negative CEGs of DUSP1 in OVCA (Supplementary Figure S5), we performed enrichment analysis. As illustrated by crosstalk maps of KEGG pathways in Figure 6, downregulated positive CEGs of DUSP1 mostly participated in MAPK signalling pathways, cGMP-PKG signalling pathways, and TGF-β signalling pathways, etc., whereas a majority of upregulated negative CEGs of DUSP1 appeared in the pathways of cell cycles and oocyte meiosis (Supplementary Table S1). Similar results were also revealed via Reactome Pathway enrichment (Figure 7). GO enrichment revealed that downregulated CEGs of DUSP1 were enriched in terms related to extracellular matrix (GO: 0030198, GO: 0062023, GO: 0005201, etc.; Figure 8A); however, upregulated CEGs of DUSP1 were enriched in terms of cell cycle (GO: 0000280, GO: 0098687, GO: 0140097, etc.; Figure 8B).
FIGURE 6
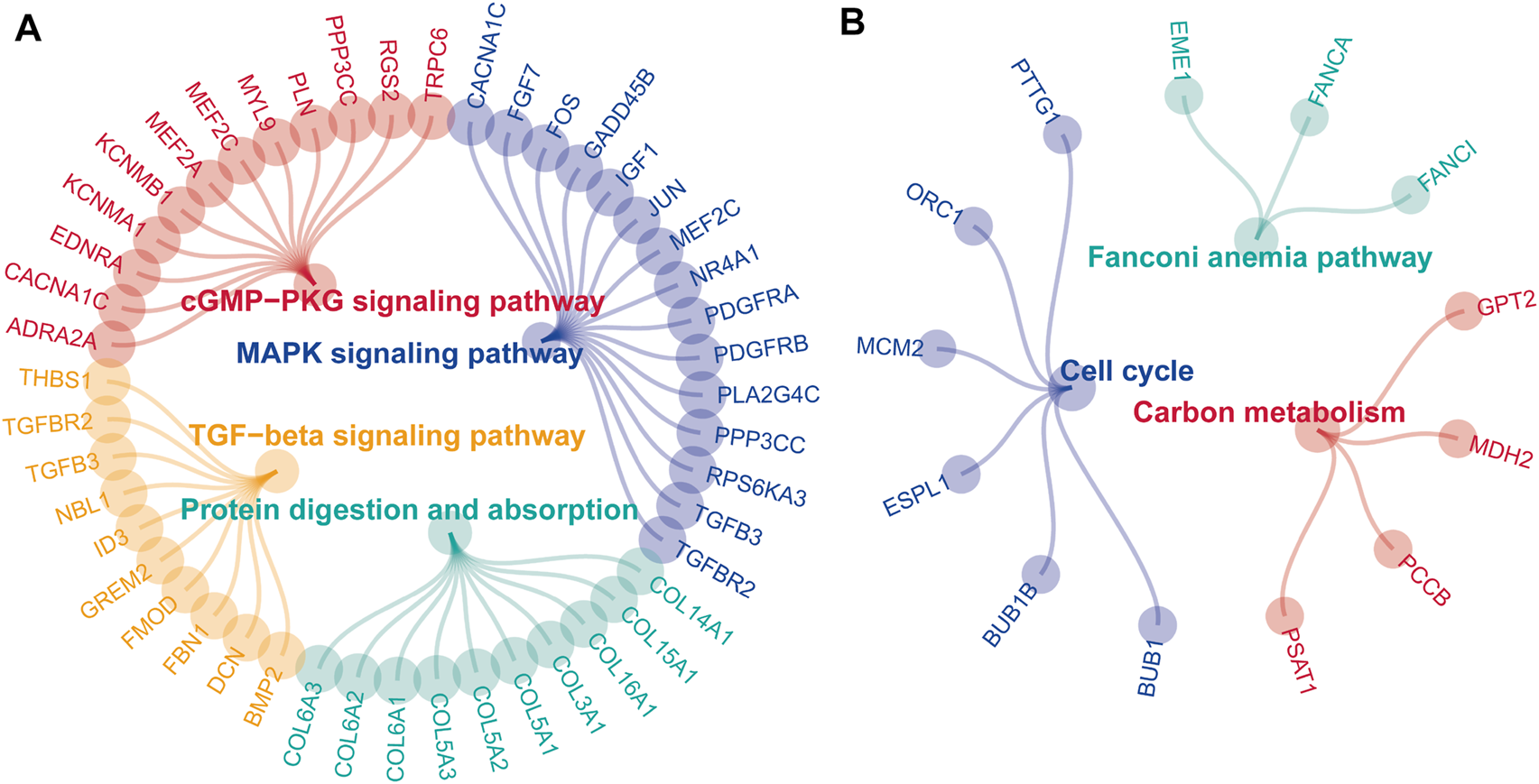
Enrichment plots of KEGG pathways of downregulated positive-correlated genes of DUSP1 (A) and upregulated negative-correlated genes of DUSP1 (B).
FIGURE 7
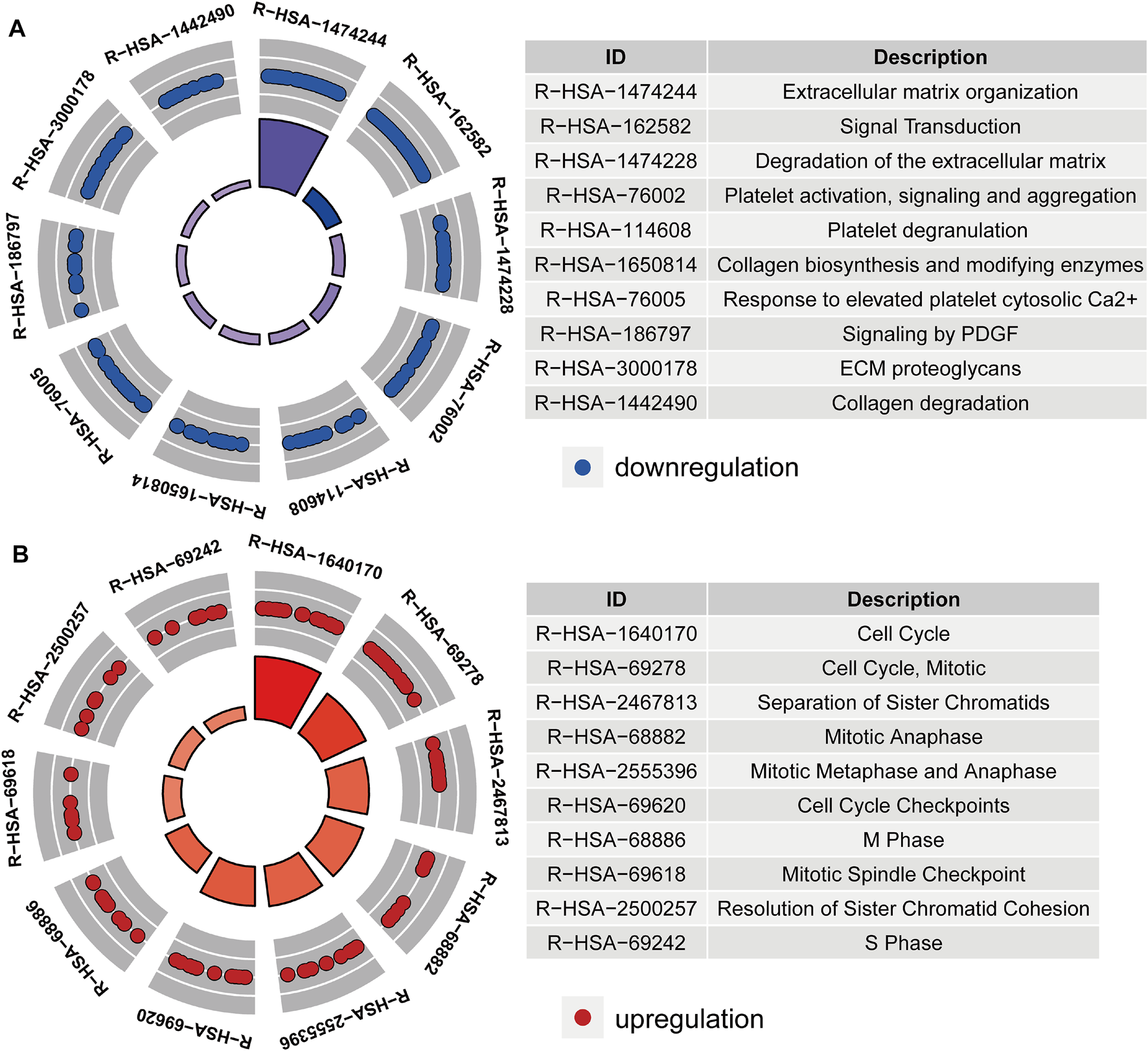
Circle plots of Reactome pathways enrichment of downregulated positive-correlated genes of DUSP1 (A) and upregulated negative-correlated genes of DUSP1 (B).
FIGURE 8
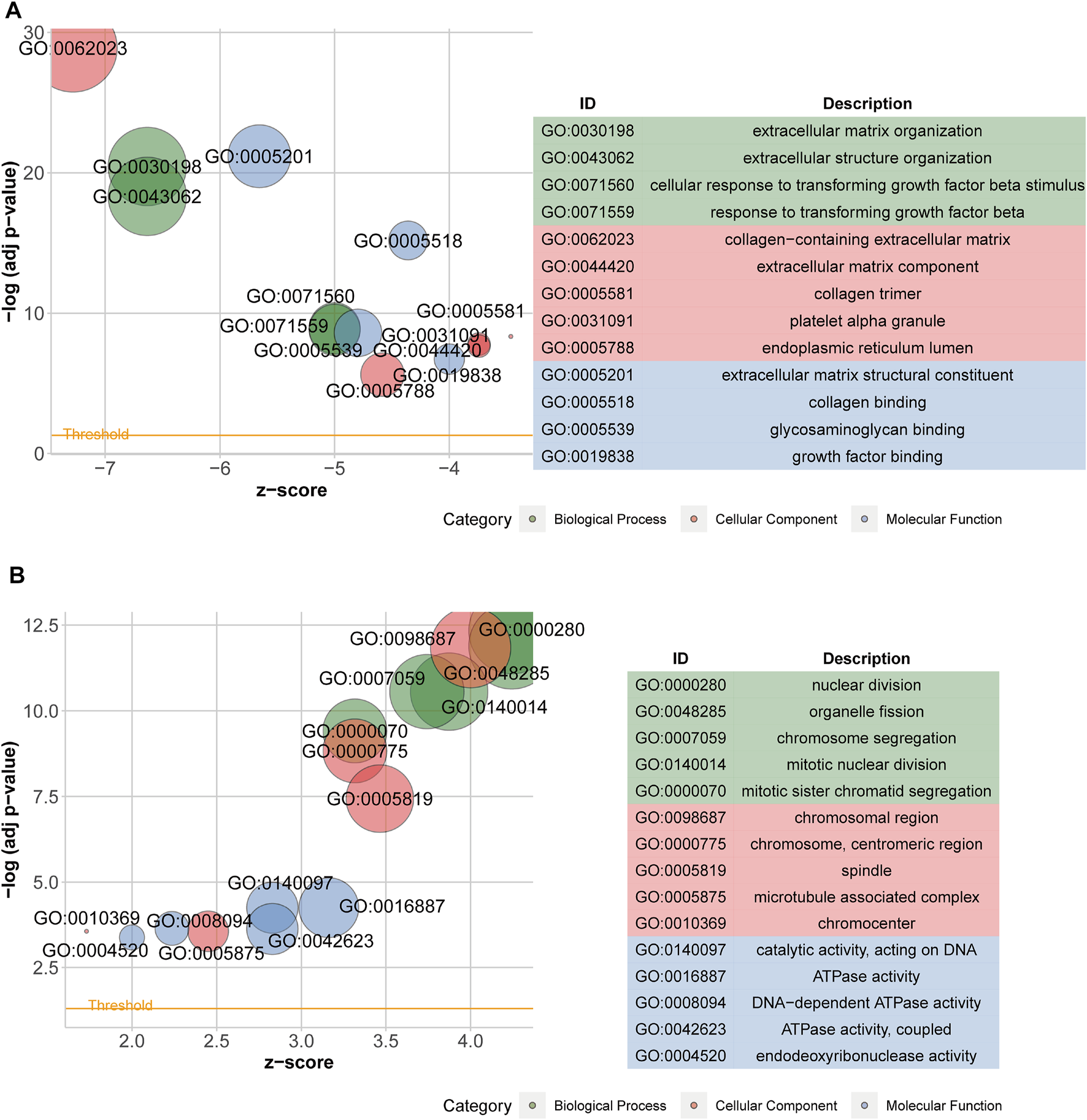
Bubble plots of GO enrichment analysis of downregulated positive-correlated genes of DUSP1 (A) and upregulated negative-correlated genes of DUSP1 (B).
Role of Dual-Specificity Protein Phosphatase 1 in Chemotherapy Resistance and Molecular Docking
Through ridge regression, we predicted the IC50 of cisplatin, paclitaxel, docetaxel, and gefitinib in the TCGA-OVCA cohort. The boxplots demonstrated that patients with high DUSP1 expression tended to have lower IC50 of cisplatin, paclitaxel, and gefitinib than patients with low DUSP1 expression (Figures 9A,B,D); this indicated that OVCA patients with higher DUSP1 expression may respond better to cisplatin, paclitaxel, and gefitinib than patients with lower DUSP1 expression. By contrast, patients with high DUSP1 expression tended to have higher IC50 of docetaxel than patients with low DUSP1 expression (Figure 9C).
FIGURE 9
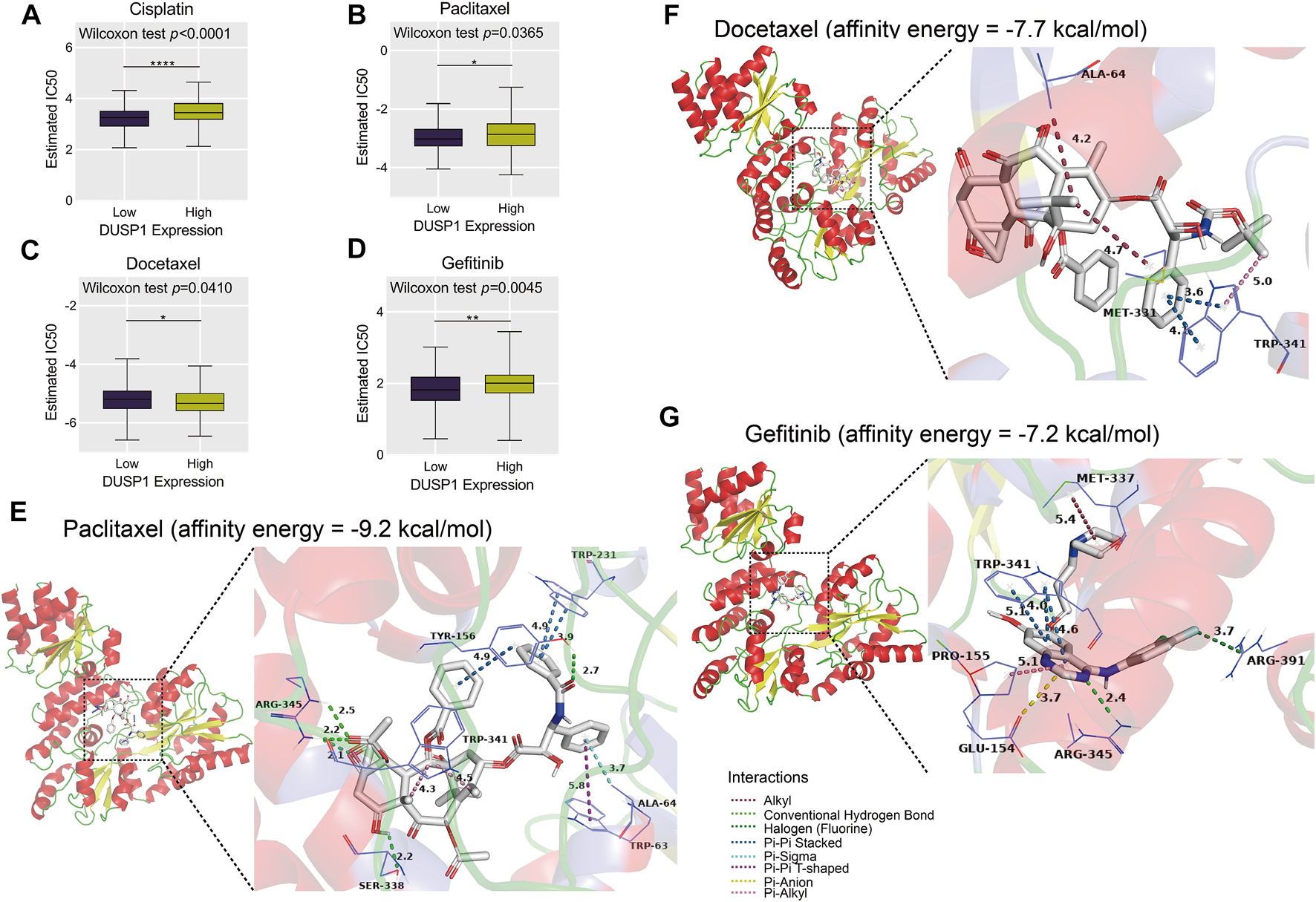
Estimated half-maximal inhibitory concentration (IC50) of cisplatin, paclitaxel, docetaxel and gefitinib in high- and low-DUSP1 group (A–D) and molecular docking models of drug-DUSP1 protein (E–G).
To further explore the interaction between the aforementioned drugs and DUSP1, molecular docking was conducted. A cisplatin-DUSP1 docking model was unsatisfactory (affinity energy = 0 kcal/mol), which meant that we did not display this docking model. The docking models paclitaxel-DUSP1 (affinity energy = −9.2 kcal/mol), docetaxel-DUSP1 (affinity energy = −7.7 kcal/mol), and gefitinib-DUSP1 (affinity energy = −7.2 kcal/mol) are included in Figures 9E–G.
Discussion
In the present study, we demonstrated that the downregulated trend of DUSP1 protein in OVCA is based on clinical case analysis via IHC experiments (n of OVCA = 75, n of non-tumour = 45). Microarrays and RNA-seq cohorts also validated a decreasing trend of DUSP1 at mRNA levels in OVCA tissues via a large sample size (n of OVCA = 936, n of non-tumour = 214), which was also observed in peripheral blood of OVCA patients (n of OVCA = 74, n of healthy women = 47). Using multiple approaches, we simultaneously explored the relationship among DUSP1, TME, and the underlying molecular pathways in OVCA.
DUSP1 may play stimulative or suppressive roles in different types of tumours [23]. Some studies have shown the aberrant expression status of DUSP1 in malignant tumours such as prostate small cell carcinoma [44], liver cancer [45], and non-small cell lung cancer [46], which may affect the therapeutic effect of chemotherapeutic drugs. Interestingly, there has not yet been a study that has identified the dysregulation of DUSP1 at mRNA or protein levels in OVCA (based on PubMed database, as of September 3, 2021). In our study, we implemented a comprehensive analysis of the expression status of DUSP1 in OVCA and first revealed the downregulation of DUSP1 in OVCA tissues at both mRNA and protein levels through examining 1,240 samples via calculating and combining SMD. Furthermore, within our study, the downregulated DUSP1 was first demonstrated in the peripheral blood of OVCA patients among 121 samples.
The clinical impact of DUSP1 was absorbing. One previous study reported the prognostic value of DUSP1 in OVCA via high-throughput cohort [31]. However, no previous study investigated the distinguishing ability or diagnostic value of DUSP1 in OVCA. In the present study, we illustrated the sROC curve, and the AUC of 0.91 indicated that DUSP1 could commendably distinguish OVCA tissues from non-tumour tissues. The ROC curve of the peripheral blood from OVCA patients also presented a compelling discriminatory capacity and favourable diagnostic value of DUSP1 mRNA (AUC = 0.9235), which has never been reported before. DUSP1 mRNA in peripheral blood might be a diagnostic biomarker of OVCA, which still need further investigation and validation.
Previously, some studies have revealed that TME changes in ovarian cancer were relevant to clinical outcomes in patients and could serve as the basis of potential therapeutic approaches [47, 48]. Appropriate immune-targeted therapy is expected to improve the prognosis of ovarian cancer patients [49, 50]. CAFs were reported to accelerate the development of malignant tumors, including OVCA [51, 52]. CAFs could excrete extracellular matrix and participate in the remodeling of extracellular matrix, which resulted in the dynamic changes of TME [53]. Some studies revealed that CAFs could impact on multiple process in TME, such as angiogenesis, invasion, migration and immunosuppression [54]. However, the roles of CAFs were heterogeneous [55]. Some subtypes of CAFs would not facilitate the invasion, proliferation and metastasis of tumor cells, which played as a inhibitor in the development of tumors [56]. Tumour-infiltrating T cells (CD4+T cells and CD8+T cells, etc.) were identified to play crucial roles in OVCA [57]. The γδT cell is a part of the innate immune system, and previous studies identified this type of T cell as potentially able to discern the diverse antigens on the membranes of tumour cells and kill them with multiple innate cytotoxicity receptors [58–60]. In our study, we constructed a TME landscape of OVCA and demonstrated that the proportion of T cells was lower in the high-DUSP1 expression group compared to the low-DUSP1 expression group, whereas CAFs showed a converse trend, suggesting that DUSP1 may have a relationship with TME in OVCA and may be a target for immunotherapy.
DUSP1 is a member of the threonine/tyrosine DUSP family. DUSP1 can dephosphorylate threonine and tyrosine and acts as an inhibitor in p38/MAPK signalling [23]. One study reported that DUSP1 negatively regulated autophagy of OVCA cells via downregulating MAPK/ERKs signalling [61]. MAPK signalling pathways were demonstrated to regulate the proliferation, invasion, and epithelial-mesenchymal transition of OVCA cells [62, 63]. Activating the cGMP-PKG pathway could result in the activation of a MAPK signalling pathway and the proliferation and metastasis of cancer cells [64, 65]. Cell cycle pathways play a crucial role in the tumorigenesis and development of OVCA, and targeting the abnormal cell cycle might facilitate the treatment of OVCA [66–68]. In our work, we illustrated the correlated genes of DUSP1 in OVCA enriched in MAPK signalling pathway, cGMP-PKG signalling pathway and cell cycle pathway through pathway enrichment analysis. This kind of work may help us learn more about the molecular mechanisms of DUSP1 in OVCA.
Chemotherapy is a common treatment for OVCA patients [69]. DUSP1 was reported to have a relationship with resistance to paclitaxel, which is also commonly used to treat OVCA [29, 70]. Cisplatin and docetaxel are also used in chemotherapy to treat OVCA, and the resistance mechanisms and the way to enhance therapeutic effects have been widely studied [71–73]. Gefitinib is an EGFR-targeting compound and is identified to promote the prognosis of lung cancer [74, 75]. Interestingly, in our study, we illustrated that DUSP1 may be related to the resistance of cisplatin, paclitaxel, docetaxel, and gefitinib through predicting IC50. Furthermore, molecular docking revealed the interactions between compounds and DUSP1 proteins. For paclitaxel, there were conventional hydrogen bonds between paclitaxel molecules and ARG-345, between SER-338 and TYR-156 residues of DUSP1; there was pi-pi interaction and stacking between paclitaxel and TYR-156 and between TRP-231 and TRP-63 (T-shaped) residues, which jointly made this paclitaxel-DUSP1 docking model stable. Pi-stacking was also identified between docetaxel and TRP-341 and gefitinib and TRP-341, which indicated that docetaxel and gefitinib may have interactions with DUSP1 proteins.
Collectively, our study demonstrated the downregulation trend of DUSP1 in OVCA tissues at both the mRNA and protein levels; this was also verified in the peripheral blood of OVCA patients. In addition, DUSP1 may act as a tumour-suppressor in OVCA bye effecting TME and inhibiting MAPK signalling pathways. Moreover, DUSP1 and paclitaxel were proved to be a commpelling combination. However, it must be acknowledged that our study still has some limitations. First, there was a lack of clinical parameters; we were unable to fully elucidate the clinicopathological significance of DUSP1 in OVCA. In addition, the underlying pathways and transcriptional regulatory mechanisms of DUSP1 still require verification with experiments in vivo and in vitro.
Conclusion
Briefly, via combining high-throughput datasets and clinical samples we illustrated that the expression of DUSP1 was lower in OVCA than that in normal ovarian tissues at both the mRNA and protein levels. This decreasing trend was also observed in the peripheral blood of OVCA patients, and it may have non-invasive diagnostic significance. The dysregulation of DUSP1 may be related to the resistance of chemotherapy, and it had a significant interaction with paclitaxel by means of molecular docking. Furthermore, we identified that DUSP1 may participate in MAPK signalling, cell cycle pathways and the regulation of CAFs.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (2020-KY-E-095) and the Ethics Committee of the Second Affiliated Hospital of Guangxi Medical University (2021-KY-0130). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Z-QL and R-QH performed data analysis and wrote the manuscipt. J-YL, Z-GH, and LS conducted immunohistochemistrical staining for the manuscript. JL and L-YZ performed literature searches for the manuscript. J-HC, S-NH, and K-LW performed data check for the manuscript. J-HZ and J-JZ gave suggestions on design for the study. GC contributed to the revision of the manuscript. All authors have read and approved the final manuscript.
Funding
The study was supported by the Fund of Future Academic Star of Guangxi Medical University (grant number: WLXSZX21117), Guangxi Medical University 2021 Undergraduate Innovation and Entrepreneurship Training Program (grant number: 202110598124), Guangxi Higher Education Undergraduate Teaching Reform Project (grant number: 2016JGA167, 2020JGA146, 2021JGA142), Guangxi Medical University Education and Teaching Reform Project (grant number: 2019XJGZ04), Guangxi Educational Science Planning Key Project (grant number: 2021B167), Guangxi Medical High-level Key Talents Training “139” Program (grant number: 2020), Guangxi Medical University Training Program for Distinguished Young Scholars (grant number: 2017), Medical Excellence Award Funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University (grant number: 2016) and Guangxi Zhuang Autonomous Region Health Commission Self-financed Scientific Research Project (grant number: Z20180979, Z20211286).
Acknowledgments
The authors would like to thank Guangxi Key Laboratory of Medical Pathology for technical supports.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.por-journal.com/articles/10.3389/pore.2022.1610404/full#supplementary-material
Supplementary Figure S1Images of normal ovarian and ovarian carcinoma tissues stained with hematoxylin-eosin (HE) and negative control.
Supplementary Figure S2Images of immunohistochemical staining of DUSP1 in prostate carcinoma tissues as positive controls.
Supplementary Figure S3Flow chart of filtering ovarian carcinoma-related high-throughput datasets.
Supplementary Figure S4Violin plots of DUSP1 expression in different histological subtypes (A), pathological grades (B) and clinical stage (C) of ovarian carcinoma.
Supplementary Figure S5Venn diagrams of intersection between differentially expressed genes in ovarian carcinoma and correlated expressed genes of DUSP1.
References
1.
SiegelRLMillerKDFuchsHEJemalA. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. 10.3322/caac.21654
2.
ShinWParkS-YKangSLimMCSeoS-S. How to Manage Synchronous Endometrial and Ovarian Cancer Patients?BMC Cancer (2021) 21(1):489. 10.1186/s12885-021-08220-w
3.
AdambekovSLopaSEdwardsRPLemonLWangSTaylorSEet alSurvival and Recurrence after Intraperitoneal Chemotherapy Use: Retrospective Review of Ovarian Cancer Hospital Registry Data. Cancer Med (2020) 9(20):7388–97. 10.1002/cam4.3340
4.
PanXBiF. A Potential Immune-Related Long Non-coding RNA Prognostic Signature for Ovarian Cancer. Front Genet (2021) 12:694009. 10.3389/fgene.2021.694009
5.
SunXLiuQHuangJDiaoGLiangZ. Transcriptome-based Stemness Indices Analysis Reveals Platinum-Based Chemo-Theraputic Response Indicators in Advanced-Stage Serous Ovarian Cancer. Bioengineered (2021) 12(1):3753–71. 10.1080/21655979.2021.1939514
6.
MaQSongJWangSHeN. MUC1 Regulates AKT Signaling Pathway by Upregulating EGFR Expression in Ovarian Cancer Cells. Pathol Res Pract (2021) 224:153509. 10.1016/j.prp.2021.153509
7.
XueBLiSJinXLiuL. Bioinformatics Analysis of mRNA and miRNA Microarray to Identify the Key miRNA-mRNA Pairs in Cisplatin-Resistant Ovarian Cancer. BMC Cancer (2021) 21(1):452. 10.1186/s12885-021-08166-z
8.
YinLWangY. Long Non-coding RNA NEAT1 Facilitates the Growth, Migration, and Invasion of Ovarian Cancer Cells via the Let-7 g/MEST/ATGL axis. Cancer Cell Int (2021) 21(1):437. 10.1186/s12935-021-02018-3
9.
GuoJZhuYYuLLiYGuoJCaiJet alAspirin Inhibits Tumor Progression and Enhances Cisplatin Sensitivity in Epithelial Ovarian Cancer. PeerJ (2021) 9:e11591. 10.7717/peerj.11591
10.
ZhuDHuangJLiuNLiWYanL. PSMC2/CCND1 axis Promotes Development of Ovarian Cancer through Regulating Cell Growth, Apoptosis and Migration. Cell Death Dis (2021) 12(8):730. 10.1038/s41419-021-03981-5
11.
FalzoneLScandurraGLombardoVGattusoGLavoroADistefanoABet alA Multidisciplinary Approach Remains the Best Strategy to Improve and Strengthen the Management of Ovarian Cancer (Review). Int J Oncol (2021) 59(1):53. 10.3892/ijo.2021.5233
12.
Segura-SampedroJJMorales-SorianoRArjona-SánchezÁCascales-CamposP. Secondary Surgical Cytoreduction Needs to Be Assessed Taking into Account Surgical Technique, Completeness of Cytoreduction, and Extent of Disease. World J Surg Onc (2020) 18(1):92. 10.1186/s12957-020-01853-4
13.
HuangXXieCTangJHeWYangFTianWet alAdipose Tissue Area as a Predictor for the Efficacy of Apatinib in Platinum-Resistant Ovarian Cancer: An Exploratory Imaging Biomarker Analysis of the AEROC Trial. BMC Med (2020) 18(1):267. 10.1186/s12916-020-01733-4
14.
GaoLPangY-YGuoX-YZengJ-JTangZ-QXiongD-Det alPolo like Kinase 1 Expression in Cervical Cancer Tissues Generated from Multiple Detection Methods. PeerJ (2020) 8:e10458. 10.7717/peerj.10458
15.
KatoMKYunokawaMBunSShimoiTYonemoriKMiyasakaNet alTreatment Strategies for Recurrent Ovarian Cancer in Older Adult Patients in Japan: A Study Based on Real-World Data. J Cancer Res Clin Oncol (2020) 146(5):1335–41. 10.1007/s00432-020-03168-z
16.
HeLBulanovaDOikkonenJHäkkinenAZhangKZhengSet alNetwork-guided Identification of Cancer-Selective Combinatorial Therapies in Ovarian Cancer. Brief Bioinformatics (2021) 22:bbab272. 10.1093/bib/bbab272
17.
MajidiANaRJordanSJDe FazioAWebbPM. Statin Use and Survival Following a Diagnosis of Ovarian Cancer: A Prospective Observational Study. Int J Cancer (2021) 148(7):1608–15. 10.1002/ijc.33333
18.
LiSYangTXiangYLiXZhangLDengS. Clinical Characteristics and Survival Outcomes of Malignant Struma Ovarii Confined to the Ovary. BMC Cancer (2021) 21(1):383. 10.1186/s12885-021-08118-7
19.
Schüler-ToprakSWeberFSkrzypczakMOrtmannOTreeckO. Expression of Estrogen-Related Receptors in Ovarian Cancer and Impact on Survival. J Cancer Res Clin Oncol (2021) 147(9):2555–67. 10.1007/s00432-021-03673-9
20.
LeanderssonPHogbergTDickmanPWMalanderSBorgfeldtC. Incidence and Survival of Epithelial Ovarian, Fallopian Tube, Peritoneal, and Undesignated Abdominal/pelvic Cancers in Sweden 1960-2014: A Population-Based Cohort Study. BMC Cancer (2021) 21(1):465. 10.1186/s12885-021-08169-w
21.
TeixeiraLACandido Dos ReisFJ. BRCA1 Expression by Immunohistochemistry and Prognosis in Ovarian Cancer: A Systematic Review and Meta-Analysis. Targ Oncol (2020) 15(1):37–46. 10.1007/s11523-020-00697-y
22.
JinQLiRHuNXinTZhuPHuSet alDUSP1 Alleviates Cardiac Ischemia/reperfusion Injury by Suppressing the Mff-Required Mitochondrial Fission and Bnip3-Related Mitophagy via the JNK Pathways. Redox Biol (2018) 14:576–87. 10.1016/j.redox.2017.11.004
23.
ShenJZhangYYuHShenBLiangYJinRet alRole of DUSP1/MKP1 in Tumorigenesis, Tumor Progression and Therapy. Cancer Med (2016) 5(8):2061–8. 10.1002/cam4.772
24.
ChenXYuCKangRKroemerGTangD. Cellular Degradation Systems in Ferroptosis. Cell Death Differ (2021) 28(4):1135–48. 10.1038/s41418-020-00728-1
25.
PragerBCVasudevanHNDixitDBernatchezJAWuQWallaceLCet alThe Meningioma Enhancer Landscape Delineates Novel Subgroups and Drives Druggable Dependencies. Cancer Discov (2020) 10(11):1722–41. 10.1158/2159-8290.cd-20-0160
26.
Martínez-MartínezDToledo LoboMVBaqueroPRoperoSAnguloJCChiloechesAet alDownregulation of Snail by DUSP1 Impairs Cell Migration and Invasion through the Inactivation of JNK and ERK and Is Useful as a Predictive Factor in the Prognosis of Prostate Cancer. Cancers (2021) 13(5):1158. 10.3390/cancers13051158
27.
DuYLuSGeJLongDWenCTanSet alROCK2 Disturbs MKP1 Expression to Promote Invasion and Metastasis in Hepatocellular Carcinoma. Am J Cancer Res (2020) 10(3):884–96.
28.
PLOS ONE Editors. Expression of Concern: DUSP1 Is a Novel Target for Enhancing Pancreatic Cancer Cell Sensitivity to Gemcitabine. PloS one (2020) 15(5):e0233098. 10.1371/journal.pone.0233098
29.
KangY-SSeokH-JJeongE-JKimYYunS-JMinJ-Ket alDUSP1 Induces Paclitaxel Resistance through the Regulation of P-Glycoprotein Expression in Human Ovarian Cancer Cells. Biochem Biophys Res Commun (2016) 478(1):403–9. 10.1016/j.bbrc.2016.07.035
30.
ZhangXSuYWuXXiaoRWuYYangBet alIntegrative Analysis of the Common Genetic Characteristics in Ovarian Cancer Stem Cells Sorted by Multiple Approaches. J Ovarian Res (2020) 13(1):116. 10.1186/s13048-020-00715-7
31.
LiLCaiSLiuSFengHZhangJ. Bioinformatics Analysis to Screen the Key Prognostic Genes in Ovarian Cancer. J Ovarian Res (2017) 10(1):27. 10.1186/s13048-017-0323-6
32.
YangYZhouJ-YZhaoL-JGaoB-RWanX-PWangJ-L. Dual-specificity Phosphatase 1 Deficiency Induces Endometrioid Adenocarcinoma Progression via Activation of Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase Pathway. Chin Med J (2016) 129(10):1154–60. 10.4103/0366-6999.181954
33.
LiangZ-QZhongLYLiJShenJHTuXYZhongZHet alClinicopathological Significance and Underlying Molecular Mechanism of Downregulation of Basonuclin 1 Expression in Ovarian Carcinoma. Exp Biol Med (2021) 247:15353702211052036. 10.1177/15353702211052036
34.
LiangZQGaoLChenJHDaiWBSuYSChenG. Downregulation of the Coiled-Coil Domain Containing 80 and its Perspective Mechanisms in Ovarian Carcinoma: A Comprehensive Study. Int J Genomics (2021) 2021:3752871. 10.1155/2021/3752871
35.
GautierLCopeLBolstadBMIrizarryRA. Affy-Analysis of Affymetrix GeneChip Data at the Probe Level. Bioinformatics (2004) 20(3):307–15. 10.1093/bioinformatics/btg405
36.
LeekJTJohnsonWEParkerHSJaffeAEStoreyJD. The Sva Package for Removing Batch Effects and Other Unwanted Variation in High-Throughput Experiments. Bioinformatics (Oxford, England) (2012) 28(6):882–3. 10.1093/bioinformatics/bts034
37.
GaoLYanS-b.YangJKongJ-l.ShiKMaF-c.et alMiR-182-5p and its Target HOXA9 in Non-small Cell Lung Cancer: A Clinical and In-Silico Exploration with the Combination of RT-qPCR, miRNA-Seq and miRNA-Chip. BMC Med Genomics (2020) 13(1):3. 10.1186/s12920-019-0648-7
38.
PangYYLiJDGaoLYangXDangYWLaiZFet alThe Clinical Value and Potential Molecular Mechanism of the Downregulation of MAOA in Hepatocellular Carcinoma Tissues. Cancer Med (2020) 9(21):8004–19. 10.1002/cam4.3434
39.
SturmGFinotelloFPetitprezFZhangJDBaumbachJFridmanWHet alComprehensive Evaluation of Transcriptome-Based Cell-type Quantification Methods for Immuno-Oncology. Bioinformatics (Oxford, England) (2019) 35(14):i436–i445. 10.1093/bioinformatics/btz363
40.
WuTHuEXuSChenMGuoPDaiZet alclusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation (2021) 2:100141. 10.1016/j.xinn.2021.100141
41.
BuDLuoHHuoPWangZZhangSHeZet alKOBAS-I: Intelligent Prioritization and Exploratory Visualization of Biological Functions for Gene Enrichment Analysis. Nucleic Acids Res (2021) 49(W1):W317–W325. 10.1093/nar/gkab447
42.
GeeleherPCoxNHuangRS. pRRophetic: An R Package for Prediction of Clinical Chemotherapeutic Response from Tumor Gene Expression Levels. PloS one (2014) 9(9):e107468. 10.1371/journal.pone.0107468
43.
TrottOOlsonAJ. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J Comput Chem (2010) 31(2):455–61. 10.1002/jcc.21334
44.
ZhangYZhangYChenMLiuCXiangC. DUSP1 Is Involved in the Progression of Small Cell Carcinoma of the Prostate. Saudi J Biol Sci (2018) 25(5):858–62. 10.1016/j.sjbs.2017.09.015
45.
ChiofaloBBruniSCertelliCSperdutiIBaioccoEVizzaE. Primary Debulking Surgery vs. Interval Debulking Surgery for Advanced Ovarian Cancer: Review of the Literature and Meta-Analysis. Minerva Med (2019) 110(4):330–40. 10.23736/S0026-4806.19.06078-6
46.
ChenZChenQChengZGuJFengWLeiTet alLong Non-coding RNA CASC9 Promotes Gefitinib Resistance in NSCLC by Epigenetic Repression of DUSP1. Cell Death Dis (2020) 11(10):858. 10.1038/s41419-020-03047-y
47.
DuYWeiNMaRJiangS-HSongD. Long Noncoding RNA MIR210HG Promotes the Warburg Effect and Tumor Growth by Enhancing HIF-1α Translation in Triple-Negative Breast Cancer. Front Oncol (2020) 10:580176. 10.3389/fonc.2020.580176
48.
BaciDBosiAGallazziMRizziMNoonanDMPoggiAet alThe Ovarian Cancer Tumor Immune Microenvironment (TIME) as Target for Therapy: A Focus on Innate Immunity Cells as Therapeutic Effectors. Int J Mol Sci (2020) 21(9):3125. 10.3390/ijms21093125
49.
OdunsiK. Immunotherapy in Ovarian Cancer. Ann Oncol (2017) 28(Suppl. l_8):viii1–viii7. 10.1093/annonc/mdx444
50.
GhisoniEImbimboMZimmermannSValabregaG. Ovarian Cancer Immunotherapy: Turning up the Heat. Int J Mol Sci (2019) 20(12):2927. 10.3390/ijms20122927
51.
MicekHMVisetsoukMRFleszarAJKreegerPK. The Many Microenvironments of Ovarian Cancer. Adv Exp Med Biol (2020) 1296:199–213. 10.1007/978-3-030-59038-3_12
52.
KalluriR. The Biology and Function of Fibroblasts in Cancer. Nat Rev Cancer (2016) 16(9):582–98. 10.1038/nrc.2016.73
53.
LiMWangJWangCXiaLXuJXieXet alMicroenvironment Remodeled by Tumor and Stromal Cells Elevates Fibroblast-Derived COL1A1 and Facilitates Ovarian Cancer Metastasis. Exp Cel Res (2020) 394(1):112153. 10.1016/j.yexcr.2020.112153
54.
YamauchiMGibbonsDLZongCFradetteJJBota-RabassedasNKurieJM. Fibroblast Heterogeneity and its Impact on Extracellular Matrix and Immune Landscape Remodeling in Cancer. Matrix Biol (2020) 91-92:8–18. 10.1016/j.matbio.2020.05.001
55.
GivelA-MKiefferYScholer-DahirelASirvenPCardonMPelonFet almiR200-regulated CXCL12β Promotes Fibroblast Heterogeneity and Immunosuppression in Ovarian Cancers. Nat Commun (2018) 9(1):1056. 10.1038/s41467-018-03348-z
56.
HussainAVoisinVPoonSKaramboulasCBuiNHBMeensJet alDistinct Fibroblast Functional States Drive Clinical Outcomes in Ovarian Cancer and are Regulated by TCF21. J Exp Med (2020) 217(8):e20191094. 10.1084/jem.20191094
57.
WangWZouWLiuJR. Tumor-infiltrating T Cells in Epithelial Ovarian Cancer: Predictors of Prognosis and Biological Basis of Immunotherapy. Gynecol Oncol (2018) 151(1):1–3. 10.1016/j.ygyno.2018.09.005
58.
EdwardsSCSuttonCELadellKGrantEJMcLarenJERocheFet alA Population of Proinflammatory T Cells Coexpresses αβ and γδ T Cell Receptors in Mice and Humans. J Exp Med (2020) 217(5):e20190834. 10.1084/jem.20190834
59.
PauzaCDLiouM-LLahusenTXiaoLLapidusRGCairoCet alGamma Delta T Cell Therapy for Cancer: It Is Good to Be Local. Front Immunol (2018) 9:1305. 10.3389/fimmu.2018.01305
60.
MorandiFYazdanifarMCoccoCBertainaAAiroldiI. Engineering the Bridge between Innate and Adaptive Immunity for Cancer Immunotherapy: Focus on γδ T and NK Cells. Cells (2020) 9(8):1757. 10.3390/cells9081757
61.
WangJZhouJ-YKhoDReinersJJWuGS. Role for DUSP1 (Dual-specificity Protein Phosphatase 1) in the Regulation of Autophagy. Autophagy (2016) 12(10):1791–803. 10.1080/15548627.2016.1203483
62.
ZhuJZhengYZhangHLiuYSunHZhangP. Galectin-1 Induces Metastasis and Epithelial-Mesenchymal Transition (EMT) in Human Ovarian Cancer Cells via Activation of the MAPK JNK/p38 Signalling Pathway. Am J Transl Res (2019) 11(6):3862–78.
63.
LiuYDingYNieYYangM. EMP1 Promotes the Proliferation and Invasion of Ovarian Cancer Cells through Activating the MAPK Pathway. Onco Targets Ther (2020) 13:2047–55. 10.2147/ott.s240028
64.
LvYWangXLiXXuGBaiYWuJet alNucleotide De Novo Synthesis Increases Breast Cancer Stemness and Metastasis via cGMP-PKG-MAPK Signaling Pathway. Plos Biol (2020) 18(11):e3000872. 10.1371/journal.pbio.3000872
65.
WangZZhangCChangJTianXZhuCXuW. LncRNA EMX2OS, Regulated by TCF12, Interacts with FUS to Regulate the Proliferation, Migration and Invasion of Prostate Cancer Cells through the cGMP-PKG Signaling Pathway. Onco Targets Ther (2020) 13:7045–56. 10.2147/ott.s243552
66.
LinWYeH. Anticancer Activity of Ursolic Acid on Human Ovarian Cancer Cells via ROS and MMP Mediated Apoptosis, Cell Cycle Arrest and Downregulation of PI3K/AKT Pathway. J BUON (2020) 25(2):750–6.
67.
WangPZhangJXiongXYuanWQinSCaoWet alIcariin Suppresses Cell Cycle Transition and Cell Migration in Ovarian Cancer Cells. Oncol Rep (2019) 41(4):2321–8. 10.3892/or.2019.6986
68.
BakhtiariHGheysarzadehAGhanadianMAghaeiM. 15-Hydroxy-8(17),13(E)-labdadiene-19-carboxylic Acid (HLCA) Inhibits Proliferation and Induces Cell Cycle Arrest and Apoptosis in Ovarian Cancer Cells. Life Sci (2021) 267:118981. 10.1016/j.lfs.2020.118981
69.
OrrBEdwardsRP. Diagnosis and Treatment of Ovarian Cancer. Hematol Oncol Clin North Am (2018) 32(6):943–64. 10.1016/j.hoc.2018.07.010
70.
KangYNagarajaASArmaiz-PenaGNDorniakPLHuWRupaimooleRet alAdrenergic Stimulation of DUSP1 Impairs Chemotherapy Response in Ovarian Cancer. Clin Cancer Res (2016) 22(7):1713–24. 10.1158/1078-0432.ccr-15-1275
71.
ZhouH-HChenXCaiL-YNanX-WChenJ-HChenX-Xet alErastin Reverses ABCB1-Mediated Docetaxel Resistance in Ovarian Cancer. Front Oncol (2019) 9:1398. 10.3389/fonc.2019.01398
72.
HuZCaiMZhangYTaoLGuoR. miR-29c-3p Inhibits Autophagy and Cisplatin Resistance in Ovarian Cancer by Regulating FOXP1/ATG14 Pathway. Cell Cycle (2020) 19(2):193–206. 10.1080/15384101.2019.1704537
73.
HuYRanMWangBLinYChengYZhengS. Co-Delivery of Docetaxel and Curcumin via Nanomicelles for Enhancing Anti-ovarian Cancer Treatment. Int J Nanomedicine (2020) 15:9703–15. 10.2147/ijn.s274083
74.
HosomiYMoritaSSugawaraSKatoTFukuharaTGemmaAet alGefitinib Alone versus Gefitinib Plus Chemotherapy for Non-small-cell Lung Cancer with Mutated Epidermal Growth Factor Receptor: NEJ009 Study. J Clin Oncol (2020) 38(2):115–23. 10.1200/jco.19.01488
75.
NoronhaVPatilVMJoshiAMenonNChouguleAMahajanAet alGefitinib versus Gefitinib Plus Pemetrexed and Carboplatin Chemotherapy in EGFR-Mutated Lung Cancer. J Clin Oncol (2020) 38(2):124–36. 10.1200/jco.19.01154
Summary
Keywords
molecular docking, dual-specificity protein phosphatase 1, immune landscape, cancer associated fibroblasts, ovarian carcinoma
Citation
Liang Z-Q, He R-Q, Luo J-Y, Huang Z-G, Li J, Zhong L-Y, Chen J-H, Huang S-N, Shi L, Wei K-L, Zeng J-H, Zeng J-J and Chen G (2022) Downregulated Dual-Specificity Protein Phosphatase 1 in Ovarian Carcinoma: A Comprehensive Study With Multiple Methods. Pathol. Oncol. Res. 28:1610404. doi: 10.3389/pore.2022.1610404
Received
26 February 2022
Accepted
23 June 2022
Published
15 July 2022
Volume
28 - 2022
Edited by
Anna Sebestyén, Semmelweis University, Hungary
Updates
Copyright
© 2022 Liang, He, Luo, Huang, Li, Zhong, Chen, Huang, Shi, Wei, Zeng, Zeng and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Chen, chengang@gxmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.